Team:TU Darmstadt/safety
From 2013.igem.org
| (3 intermediate revisions not shown) | |||
| Line 179: | Line 179: | ||
Many kill switches include promoters which are induced by the presence of a signal like IPTG or heat. Since these kill switches can malfunction due to human failure, we chose a different approach that fits perfectly to our device: A kill switch that is induced by blue light. | Many kill switches include promoters which are induced by the presence of a signal like IPTG or heat. Since these kill switches can malfunction due to human failure, we chose a different approach that fits perfectly to our device: A kill switch that is induced by blue light. | ||
| - | <i>Ohlendorf et al.</i><sup><span style="color:blue"> [1]</span></sup> constructed the pDawn vector which contains the blue light sensitive histidine kinase YF1. In the presence of blue light YF1 does not phosphorylate its cognate response regulator FixJ which then does not drive gene expression from the FixK2 promoter. Downstream of the promoter the λ phage repressor cI is located which represses the strong λ phage promotor pR. | + | <i>Ohlendorf et al.</i><sup><span style="color:blue"> <font color="#F0F8FF" face="Arial regular">[1]</font></span></sup> constructed the pDawn vector which contains the blue light sensitive histidine kinase YF1. In the presence of blue light YF1 does not phosphorylate its cognate response regulator FixJ which then does not drive gene expression from the FixK2 promoter. Downstream of the promoter the λ phage repressor cI is located which represses the strong λ phage promotor pR. |
<br> | <br> | ||
| - | < | + | <iframe width="320" height="240" src="//www.youtube.com/embed/9sIxNTcWG3o" frameborder="0" allowfullscreen></iframe> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | <img alt="safety" src="/wiki/images/2/27/Liks_schema.png" width=" | + | |
| + | <img alt="safety" src="/wiki/images/2/27/Liks_schema.png" width="60%" height="60%" align="right"> | ||
<br> | <br> | ||
| - | Downstream of this promotor lies our toxin of choice, PezT, which was characterized by <i>Mutschler et al.</i><sup><span style="color:blue"> [2]</span></sup> to be a very strong inhibitor of cell growth. PezT is a kinase that phosphorylates uridine diphosphate-<i>N</i>-acetylglucosamine (UNAG) and is part of a pneumococcal toxin-antitoxin system which induces cell death under stress conditions. UNAG is an essential precursor in the peptidoglycan biosynthesis and phosphorylation of UNAG inhibits in <i>E. coli</i> cell wall synthesis in such an effective manner that studies failed in the past only because of its toxicity. | + | Downstream of this promotor lies our toxin of choice, PezT, which was characterized by <i>Mutschler et al.</i><sup><span style="color:blue"> <font color="#F0F8FF" face="Arial regular">[2]</font></span></sup> to be a very strong inhibitor of cell growth. PezT is a kinase that phosphorylates uridine diphosphate-<i>N</i>-acetylglucosamine (UNAG) and is part of a pneumococcal toxin-antitoxin system which induces cell death under stress conditions. UNAG is an essential precursor in the peptidoglycan biosynthesis and phosphorylation of UNAG inhibits in <i>E. coli</i> cell wall synthesis in such an effective manner that studies failed in the past only because of its toxicity. |
</p></font> | </p></font> | ||
</body> | </body> | ||
Latest revision as of 03:48, 5 October 2013
Why biosafety?
 One key issue for the implementation of Synthetic Biology in everyday life is safety. Since genetically modified organisms (GMOs) can interact with natural organisms as well as evolve and adapt to their environment, completely new approaches are needed to address scientific and public safety concerns. Safety measurements have to work independently from the operator’s skills or background knowledge.
One key issue for the implementation of Synthetic Biology in everyday life is safety. Since genetically modified organisms (GMOs) can interact with natural organisms as well as evolve and adapt to their environment, completely new approaches are needed to address scientific and public safety concerns. Safety measurements have to work independently from the operator’s skills or background knowledge.
A light-induced kill switch
Many kill switches include promoters which are induced by the presence of a signal like IPTG or heat. Since these kill switches can malfunction due to human failure, we chose a different approach that fits perfectly to our device: A kill switch that is induced by blue light.
Ohlendorf et al. [1] constructed the pDawn vector which contains the blue light sensitive histidine kinase YF1. In the presence of blue light YF1 does not phosphorylate its cognate response regulator FixJ which then does not drive gene expression from the FixK2 promoter. Downstream of the promoter the λ phage repressor cI is located which represses the strong λ phage promotor pR.
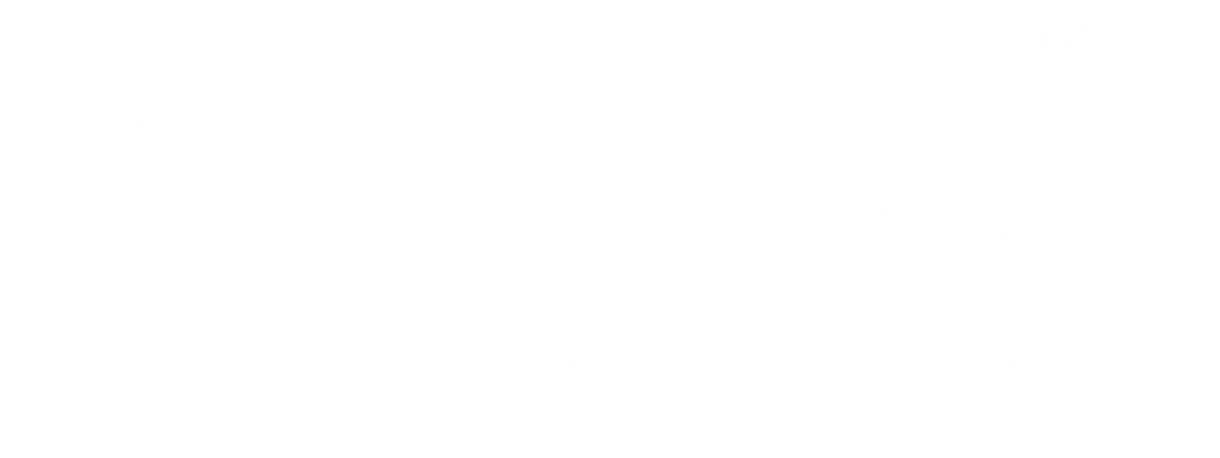
Downstream of this promotor lies our toxin of choice, PezT, which was characterized by Mutschler et al. [2] to be a very strong inhibitor of cell growth. PezT is a kinase that phosphorylates uridine diphosphate-N-acetylglucosamine (UNAG) and is part of a pneumococcal toxin-antitoxin system which induces cell death under stress conditions. UNAG is an essential precursor in the peptidoglycan biosynthesis and phosphorylation of UNAG inhibits in E. coli cell wall synthesis in such an effective manner that studies failed in the past only because of its toxicity.
What happens after a spill?
In the absence of blue light (or day light respectively) the PezT toxin is not expressed and the bacteria are alive. After the FRET measurement or a spilling the expression of PezT is induced by the blue light used in the FRET measurement or day light. Even if the spill remains unbeknownst to the operator, the expression of the toxin will be induced by day light and the leaking bacteria will contain themselves. Used capsules can be disposed without previous autoclaving. All these safety measurements raise our device’s applicability and operability in everyday life so that our device can be handled theoretically by untrained workers without any increase of risk.
 Visit our lab book
Visit our lab bookSafety form
Safety form were approved on October 3, 2013 by Evan Appleton.
 "
"