|
|
| (5 intermediate revisions not shown) |
| Line 10: |
Line 10: |
| | These experiments show that two cargo transport strategies are possible: an external labeling (the one we used with CY5) or an internal loading (with FITC-Dextran and rGFP). | | These experiments show that two cargo transport strategies are possible: an external labeling (the one we used with CY5) or an internal loading (with FITC-Dextran and rGFP). |
| | | | |
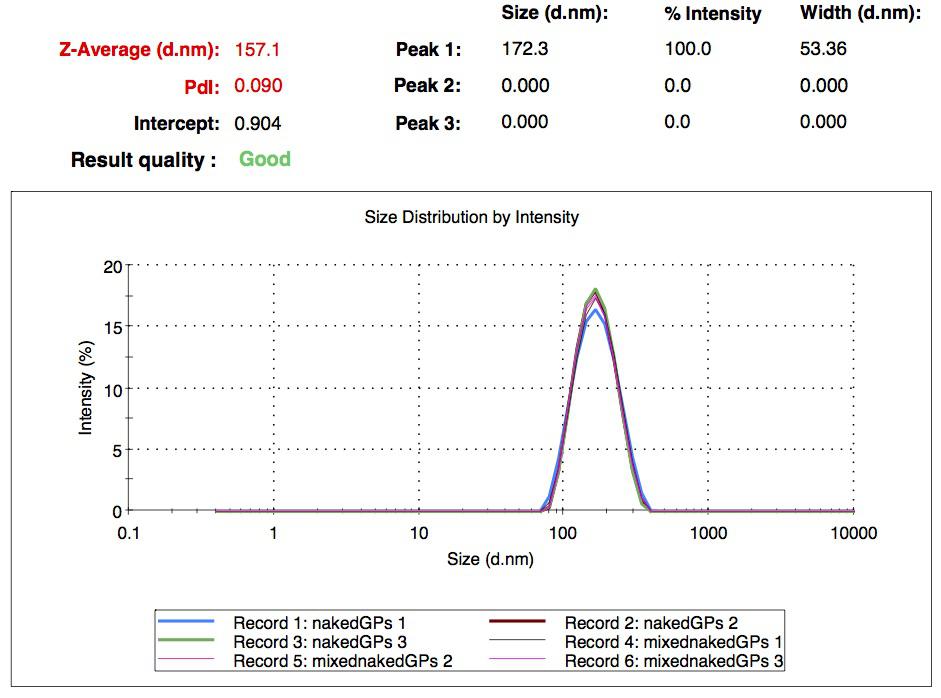
| - | [[Image:Team-EPF-Lausanne_results_NPs_DLS1.jpg|thumb|240px|left|Figure 1: DLS experiment results of the first nanoparticles we obtained: their mean diameter is a bit below 200 nm.]] | + | [[Image:Team-EPF-Lausanne_results_NPs_DLS1.jpg|thumb|250px|left|Figure 1: DLS experiment results of the first nanoparticles we obtained: their mean diameter is a bit below 200 nm.]] |
| | | | |
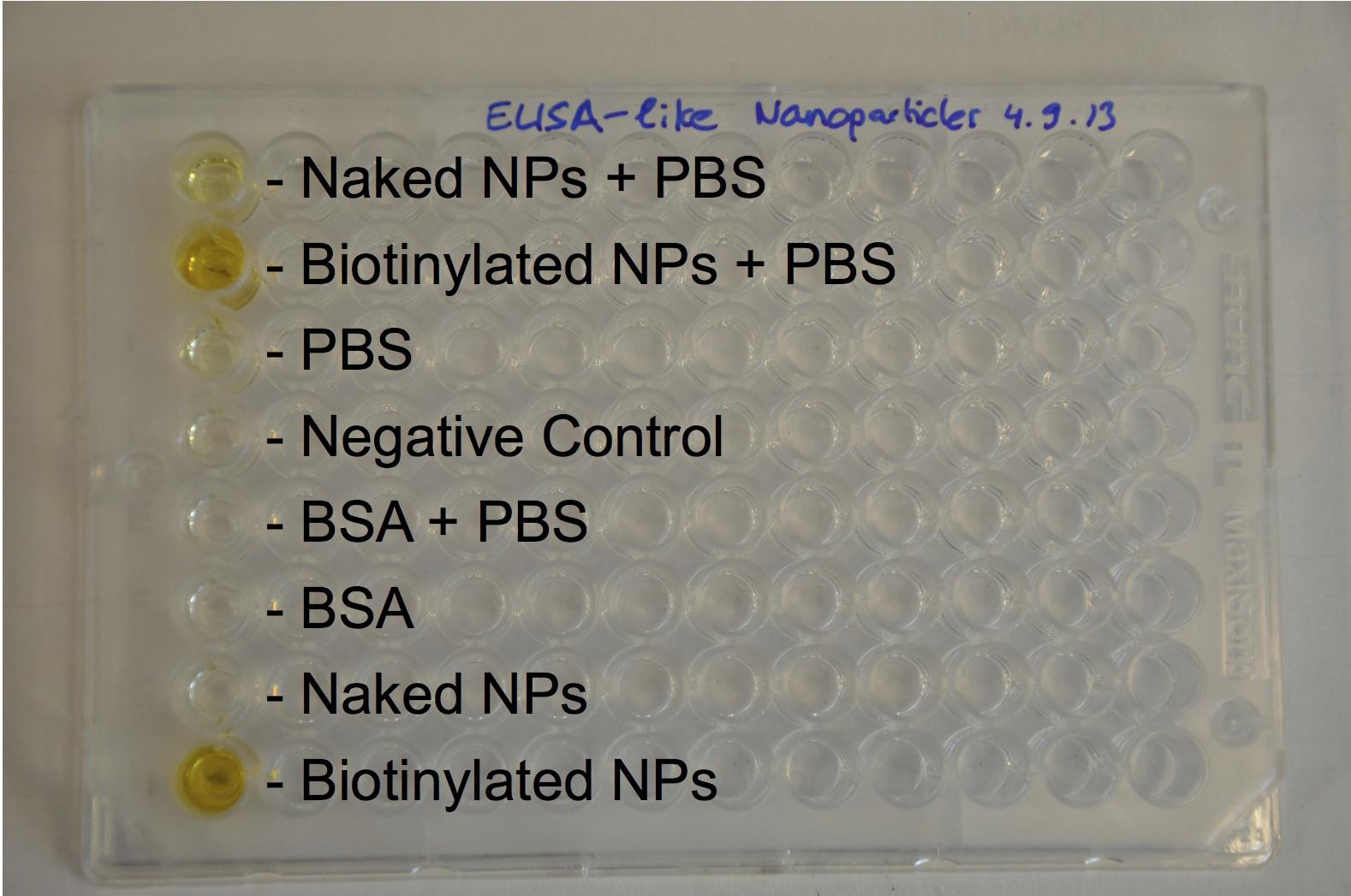
| - | [[Image:Team-EPF-Lausanne_results_ELISA-like2.jpg|thumb|240px|left|Figure 2: ELISA-like assay. The high absorbance in wells B and H (containing biotinylated nanoparticles) was quantitatively detected using a plate reader. It showed that the nanoparticles were well biotinylated.]] | + | [[Image:Team-EPF-Lausanne_results_ELISA-like2.jpg|thumb|250px|left|Figure 2: ELISA-like assay. The high absorbance in wells B and H (containing biotinylated nanoparticles) was quantitatively detected using a plate reader. It showed that the nanoparticles were well biotinylated.]] |
| | | | |
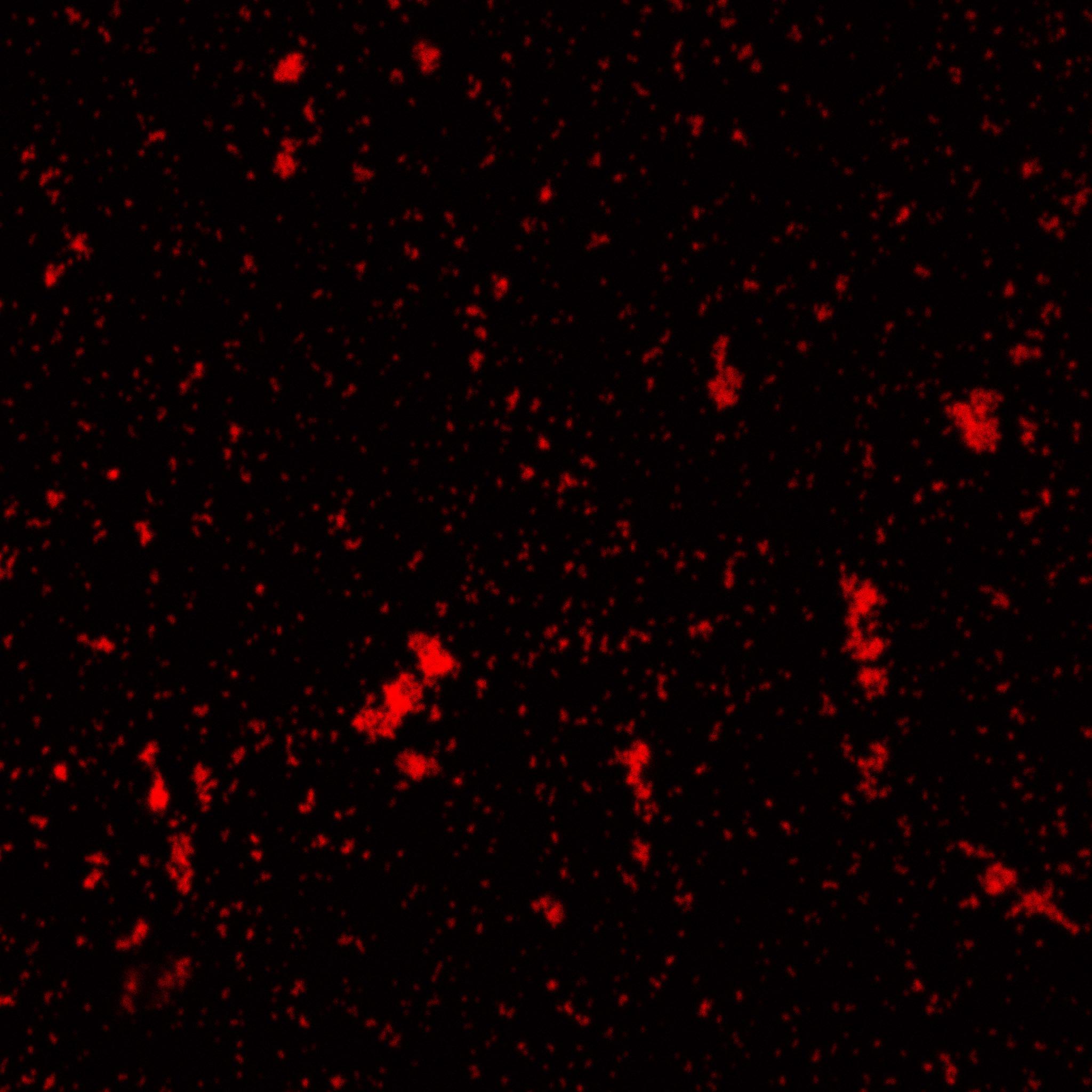
| - | [[Image:Team-EPF-Lausanne_results_confocal_positive.jpg|thumb|240px|left| Figure 3: Confocal microscopy image showing outer CY5 labeling of the nanoparticles. The size of those nanoparticles is around 200 nm, which corresponds to the previous DLS characterization.]] | + | [[Image:Team-EPF-Lausanne_results_confocal_positive.jpg|thumb|200px|left| Figure 3: Confocal microscopy image showing outer CY5 labeling of the nanoparticles. The size of those nanoparticles is around 200 nm, which corresponds to the previous DLS characterization.]] |
| | | | |
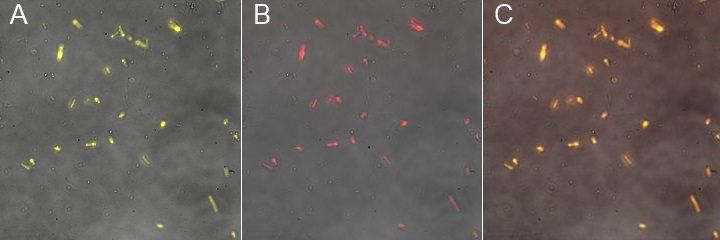
| | [[Image:Team-EPF-Lausanne_results_NPs_FITC.jpg|thumb|240px|left| Figure 4: Fluorescent microscopy of the FITC-Dextran loaded nanoparticles. In contrast to the rGFP-loaded ones, their cargo stay inside. Those nanoparticles have been successfully characterized and biotinylated.]] | | [[Image:Team-EPF-Lausanne_results_NPs_FITC.jpg|thumb|240px|left| Figure 4: Fluorescent microscopy of the FITC-Dextran loaded nanoparticles. In contrast to the rGFP-loaded ones, their cargo stay inside. Those nanoparticles have been successfully characterized and biotinylated.]] |
| Line 36: |
Line 36: |
| | | | |
| | '''Expression of streptavidin at the cell surface:''' | | '''Expression of streptavidin at the cell surface:''' |
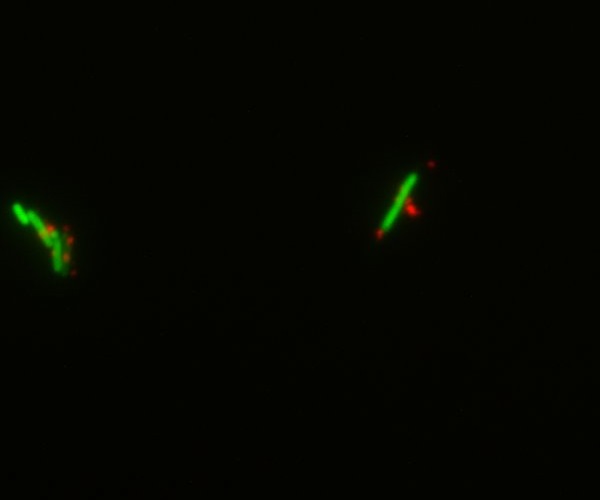
| - | Gibson assemblies of the three different streptavidin constructs worked and sequencing results matched with what expected. The growth curve of transformed E.Coli showed delayed growth, but bacteria still divide with an acceptable rate. The assay with a fluorescent biotin supposed to bind streptavidin gave some positive results (some bacteria appeared flurescent when excited at the corresponding wavelenght) but since the negative control also showed fluorescence, nothing could be proved. However, a Western blot against streptavidin showed bands at the expected size around 50 kDa of streptavidin, proving that it was expressed. | + | Gibson assemblies of the three different streptavidin constructs worked and sequencing results matched with what expected. The growth curve of transformed E.Coli showed delayed growth, but bacteria still divide with an acceptable rate. The assay with a fluorescent biotin supposed to bind streptavidin gave some positive results (some bacteria appeared fluorescent when excited at the corresponding wavelength) but since the negative control also showed fluorescence, nothing could be proved. However, a Western blot against streptavidin showed bands at the expected size around 50 kDa of streptavidin, proving that it was expressed. |
| | | | |
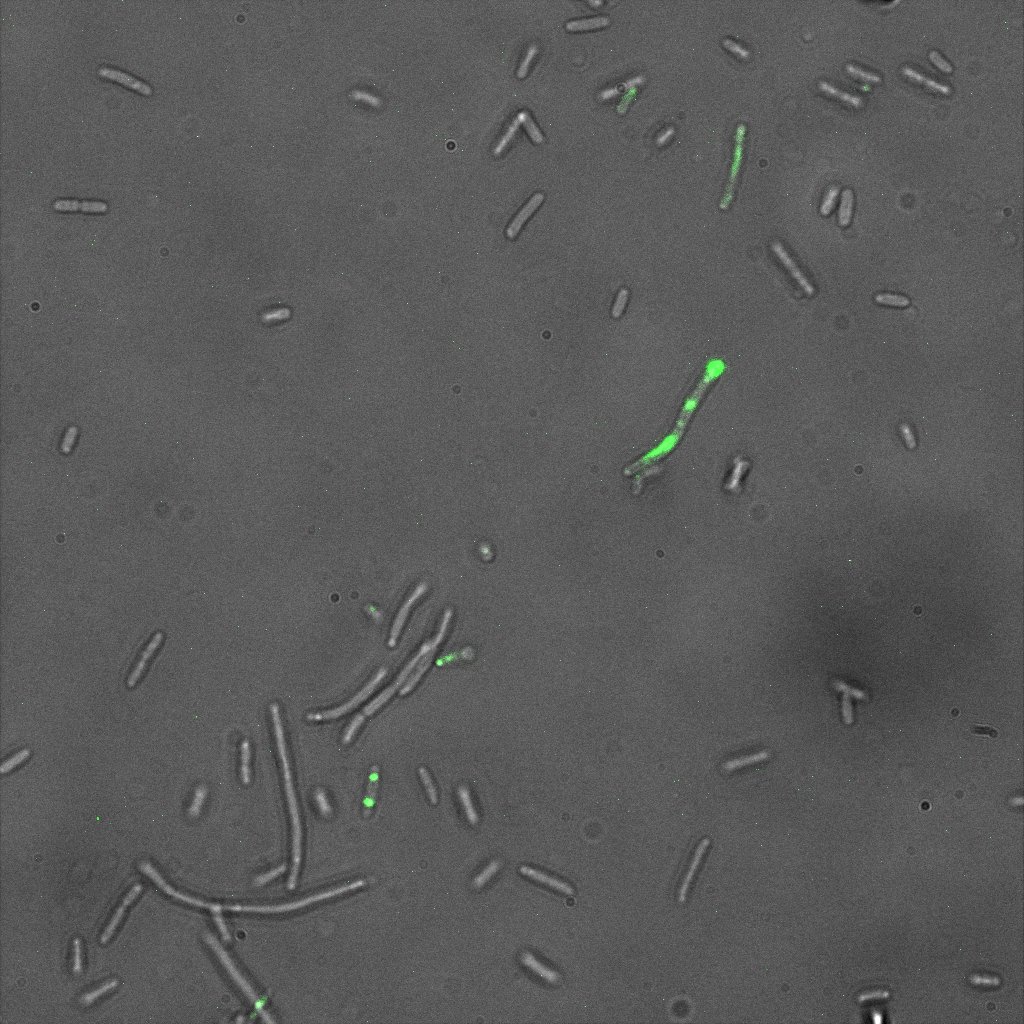
| | [[Image:Team-EPF-Lausanne_ISA5_biotin_3.10(RGB).jpg|thumb|300px|left| Figure 7: INP-streptavidin expressing cells from INP-strepta alive construct. Detection was made using fluorescently labeled biotin (green). Note that some cells were also positive on the negative control (competent cells), though they were less numerous.]] | | [[Image:Team-EPF-Lausanne_ISA5_biotin_3.10(RGB).jpg|thumb|300px|left| Figure 7: INP-streptavidin expressing cells from INP-strepta alive construct. Detection was made using fluorescently labeled biotin (green). Note that some cells were also positive on the negative control (competent cells), though they were less numerous.]] |
| - | [[Image:Team-EPF-Lausanne_WB1.jpg|thumb|400px|left| Figure 8: Western blot made from total protein of INP-strepta (from all three constructs) transformed cells. Though there is much unspecific noise, there are bands at the right size.]] | + | [[Image:Team-EPF-Lausanne_WB1.jpg|thumb|375px|left| Figure 8: Western blot made from total protein of INP-strepta (from all three constructs) transformed cells. Though there is much unspecific noise, there are bands at the right size.]] |
| | | | |
| | <br><br><br><br><br><br><br><br><br><br><br><br><br> | | <br><br><br><br><br><br><br><br><br><br><br><br><br> |
| Line 46: |
Line 46: |
| | ==Sensing:== | | ==Sensing:== |
| | We inserted two pH-dependent and one constitutive promoter in front of a superfolded GFP sequence ( [http://parts.igem.org/Part:BBa_I746908 Biobrick BBa_I746916, Main page] ). All three promoters as well as the respective backbones were successfully isolated and amplified by PCR. | | We inserted two pH-dependent and one constitutive promoter in front of a superfolded GFP sequence ( [http://parts.igem.org/Part:BBa_I746908 Biobrick BBa_I746916, Main page] ). All three promoters as well as the respective backbones were successfully isolated and amplified by PCR. |
| - | The Gibson assemblies also worked and the sequencing results showed a 100% match between the inserted promoters and the refererence sequence.<BR> | + | The Gibson assemblies also worked and the sequencing results showed a 100% match between the inserted promoters and the reference sequence.<BR> |
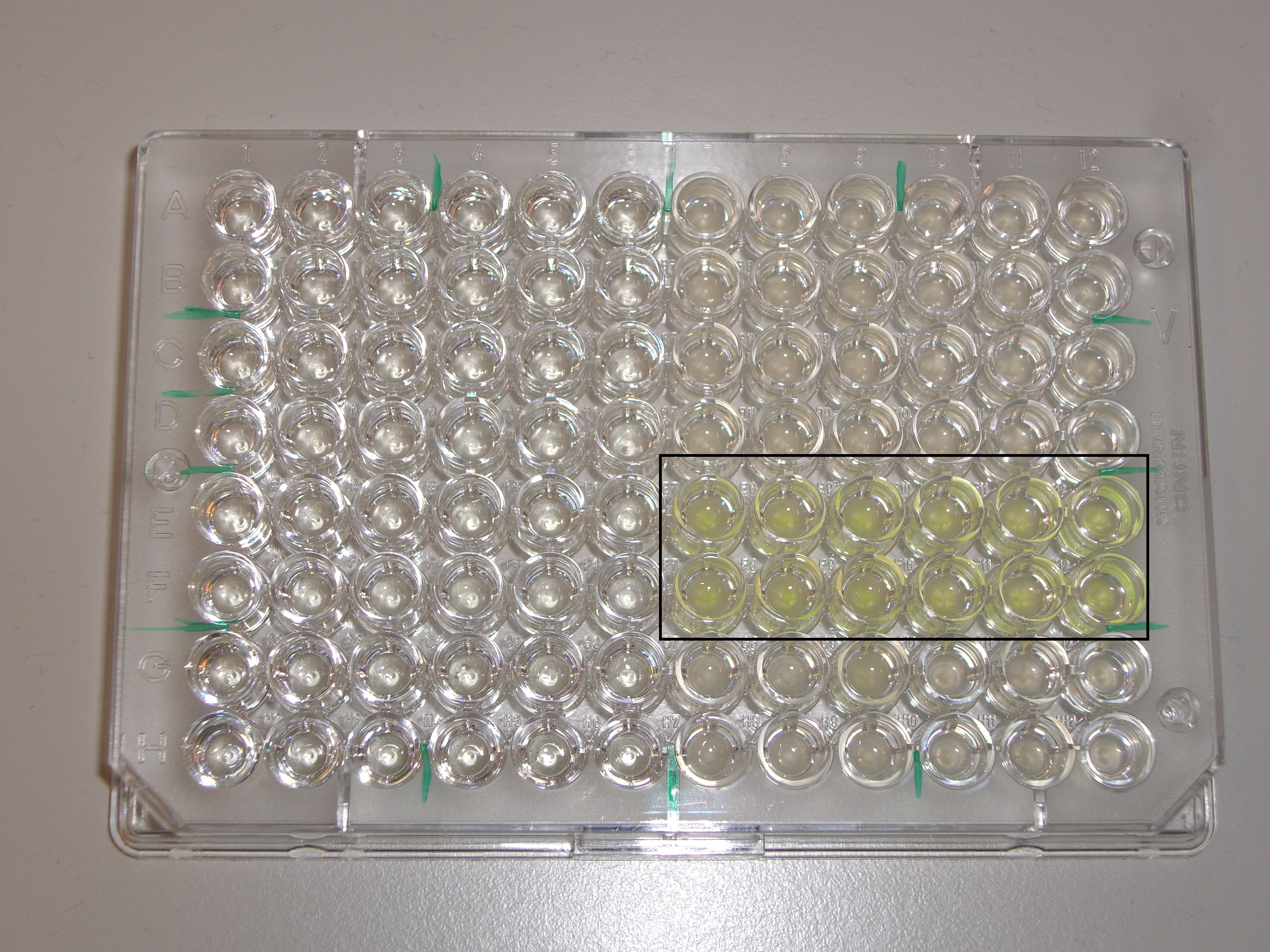
| - | Fluorescence measurements with the PlateReader were not conclusive since a lot of cells died in acidic pH, supposed to activate the promoter. However, even if we were not able to prove that acidic pH triggers expression of GFP, fluorescence could be seen under the microscope. This indicated that the promoters are functional. The constitutive promoter worked as expected, inducing the expression of superfolded GFP strongly. We used the fact that those cells' fluorescence could be seen by the naked eye and put the plasmid in our human practice kit ([https://2013.igem.org/Team:EPF_Lausanne/Kit Our Kit ]). | + | Fluorescence measurements with the Plate Reader were not conclusive since a lot of cells died in acidic pH, supposed to activate the promoter. However, even if we were not able to prove that acidic pH triggers expression of GFP, fluorescence could be seen under the microscope. This indicated that the promoters are functional. The constitutive promoter worked as expected, inducing the expression of superfolded GFP strongly. We used the fact that those cells' fluorescence could be seen by the naked eye and put the plasmid in our human practice kit ([https://2013.igem.org/Team:EPF_Lausanne/Kit Our Kit ]). |
| | [[Image: Team-EPFL-Lausanne PCR_1.1+1.2+1.3_BB.jpg|thumb|200px|left|Figure 9: 0.8% Gel, PCRs of the three backbones ]] | | [[Image: Team-EPFL-Lausanne PCR_1.1+1.2+1.3_BB.jpg|thumb|200px|left|Figure 9: 0.8% Gel, PCRs of the three backbones ]] |
| - | [[Image: Team-EPFL-Lausanne PlateReader.jpg|thumb|500px|right|Figure 10: Plate used for the PlateReader experiment. The cell marked in a black rectangle are the ones containing the constitutive promoter.]]<br><br><br><br><br> | + | [[Image: Team-EPFL-Lausanne PlateReader.jpg|thumb|500px|right|Figure 10: Plate used for the Plate Reader experiment. The cell marked in a black rectangle are the ones containing the constitutive promoter.]]<br><br><br><br><br> |
| | | | |
| | | | |
| Line 57: |
Line 57: |
| | | | |
| | ==Effector:== | | ==Effector:== |
| - | With the exception of MMP9, the Gibson Assemblies worked for both Gelatinase GelE and MMP2. The primers that were designed had introduced a stop codon upstream of GFP. But since we had planned to do constructs with and without GFP attached to the gelatinase, we continued our experiments. The Western Blot with an anti-His tag antibody did not work. The His-Tag it may be hidden in the protein, contain a mutaion or could simply not bind to the nickel columns as they were stored improperly. | + | With the exception of MMP9, the Gibson Assemblies worked for both Gelatinase GelE and MMP2. The primers that were designed had introduced a stop codon upstream of GFP. But since we had planned to do constructs with and without GFP attached to the gelatinase, we continued our experiments. The Western Blot with an anti-His tag antibody did not work. The His-Tag may be hidden in the protein, contain a mutation or could simply not bind to the nickel columns as they were stored improperly. |
| | <br> | | <br> |
| | | | |
| | ==Overall:== | | ==Overall:== |
| - | Globally, we can say that our cloning was successful. Most of the Gibson assemblies worked and we had no particular troubles growing the resulting transformed bacteria. The most delicate part was the characterization of our parts with functionnal assays. Though some experiments didn't allow us to make conclusions, a lot of parts showed encouraging results. They would maybe need to be studied in more detail for further improvement. The nanoparticle module worked very well. Our positive results would allow to try to load real drugs in a next nanoparticles batch. | + | Globally, we can say that our cloning was successful. Most of the Gibson assemblies worked and we had no particular troubles growing the resulting transformed bacteria. The most delicate part was the characterization of our parts with functional assays. Though some experiments didn't allow us to make conclusions, a lot of parts showed encouraging results. They would maybe need to be studied in more detail for further improvement. The nanoparticle module worked very well. Our positive results would allow to try to load real drugs in a next nanoparticles batch. |
| | This project was ambitious and was almost achieved, and we are really proud to share our experience with the iGEM community! | | This project was ambitious and was almost achieved, and we are really proud to share our experience with the iGEM community! |
| | | | |
| | | | |
| | {{Template:EPFL2013Footer}} | | {{Template:EPFL2013Footer}} |
 "
"