Team:BYU Provo/Notebook/Phage Purification/Fallexp/Period3/Dailylog
From 2013.igem.org
| (2 intermediate revisions not shown) | |||
| Line 75: | Line 75: | ||
The PCR products were stored in the Cholera QS II box in the freezer. | The PCR products were stored in the Cholera QS II box in the freezer. | ||
| + | |||
| + | [[File:PCR gel cloning.jpg]] | ||
AB DL AC | AB DL AC | ||
| - | |||
<br> | <br> | ||
| Line 84: | Line 85: | ||
-Today we ran PCR again on T4 wild type and mutant phage to determine the sequence differences in the capsid proteins. | -Today we ran PCR again on T4 wild type and mutant phage to determine the sequence differences in the capsid proteins. | ||
| - | :: - [[Team:BYU_Provo/Notebook/Phage_Purification/Fallexp/Period1/Exp/10.25T4PCR|10. | + | :: - [[Team:BYU_Provo/Notebook/Phage_Purification/Fallexp/Period1/Exp/10.25T4PCR|10.25 T4 PCR]] |
| + | |||
| + | AB DL AC | ||
| + | |||
| + | <br> | ||
| + | |||
| + | <font size="4"> '''10/28/13''' </font> | ||
| + | |||
| + | - We ran another PCR attempting to get bands for sequencing both the major and minor capsid proteins. We used our normal protocol except did not boil the phage this time. Skip also used the protocol he normally uses to see if reagents are the problem with us not getting a band. | ||
| + | |||
| + | [[File:T4 gel Oct 28.jpg]] | ||
| + | |||
| + | - We sent in the major and minor capsid protein genes of the wild type and mutant for sequencing. | ||
AB DL AC | AB DL AC | ||
Latest revision as of 03:22, 29 October 2013
| ||
|
|
10/21/13 -Today we ran PCR on T4 wild type and mutant phage to determine the sequence differences in the capsid proteins. AB DL AC
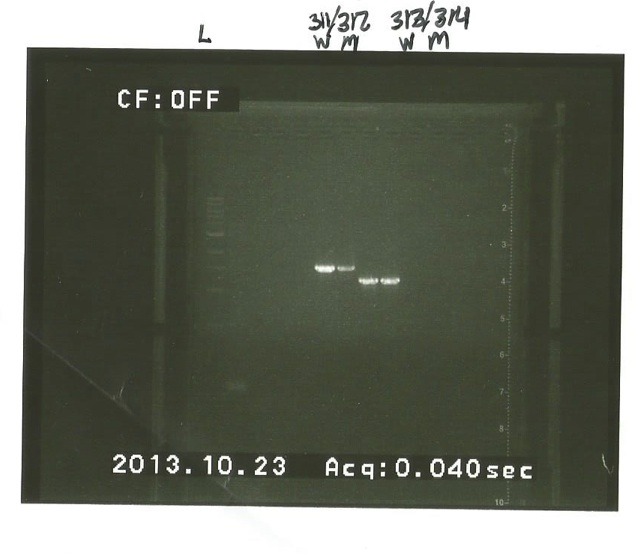
10/23/13 -Ran a gel on the PCR we did last time.
We place the samples in the order: DNA ladder, 297/298 W M, 301/302 W M, 311/312 W M, 313/314 W M W=wildtype M=mutant We got bands for both the 311/312 and 313/314 wild type and mutant. We need to do PCR cleanup and start with the cloning procedure. 297/298 and 301/302 didn't show any bands for either the wild type or the mutant. We suspect this may be to boiling them for too long (12 min) at the start. We are going to try boiling them for only 5 minutes and see if we can get bands next time. The PCR products were stored in the Cholera QS II box in the freezer. AB DL AC
10/25/13 -Today we ran PCR again on T4 wild type and mutant phage to determine the sequence differences in the capsid proteins. AB DL AC
10/28/13 - We ran another PCR attempting to get bands for sequencing both the major and minor capsid proteins. We used our normal protocol except did not boil the phage this time. Skip also used the protocol he normally uses to see if reagents are the problem with us not getting a band. - We sent in the major and minor capsid protein genes of the wild type and mutant for sequencing. AB DL AC
| |
 "
"