Phage Purification
From 2013.igem.org
(→May) |
(→5/24/13) |
||
| (27 intermediate revisions not shown) | |||
| Line 522: | Line 522: | ||
===5/14/13=== | ===5/14/13=== | ||
| + | |||
| + | AC 5/14/13 | ||
| + | |||
| + | Today I came in and infected 24 mL of LB with 1 mL of w3310 and put in the 37C incubator overnight. | ||
| + | |||
| + | I also infected 25 mL of w3110 with 100 µL of t4 phage and left overnight in the 37C incubator overnight. | ||
===5/15/13=== | ===5/15/13=== | ||
| + | |||
| + | AC 5/15/13 | ||
| + | |||
| + | We added 2.5 ml of LB to the 24 mL of t4 infected W3110 to make a final volume of 25 mL. | ||
| + | |||
| + | We added 92.5 µL of DNAse I and 12.5 µL of RNAse A into the 25mL of t4 infected w3110 Ecoli and the t7 infected BL21. | ||
| + | |||
| + | We then added 50 µL of chloroform to each lysate and let it incubate for 30 min at room temperature. | ||
| + | |||
| + | We then added .73 g of solid NaCl to both lysates and let it sit at 4 C for 1 hour. | ||
| + | |||
| + | AB 5/15/13 | ||
| + | |||
| + | To make the W3110 with T4 a total volume of 25 mL, we added 2.5 mL to the 22.5 mL of lysate. Then we added 92.5 microL DNase 1 and 12.5 RNase A to each lysate (BL21, T7 and W3110, T4; 25 mL of each) and then added 50 microL Chloroform. We let this incubate for 30 min at room temperature. | ||
| + | Next, we added .73 g solid NaCl and let the lysates sit at 4 C for 1 hour. (start 3:22, finish 4:22) | ||
| + | Then, we centrifuged at 6500 rpm (7000xg) for 10 min. We removed the phage containing supernatant and put it in a clean tube. (24 mL W3110; 25mL BL21) | ||
| + | After, we added PEG 8000 to a final concentration of 8-10% and let it stand for 1 hour. | ||
| + | W3110 - 2.4g PEG BL21 - 2.5 g PEG | ||
| + | |||
| + | DL 05/15 | ||
| + | |||
| + | Added 2.5 mL of LB to the 22.5 mL of T4 infected W3110 to make a final volume of 25 mL. | ||
| + | Added 92.5 microL of DNase I and 12.5 microL of RNase A to both T4 and T7. | ||
| + | Added 50 microL of chloroform and incubated at room temp for 20 min. | ||
| + | Added .73 g solid NaCl and let sit at 4 C for 1 hour | ||
| + | Centrifuged at 6500 rpm (7000 g) for 10 minutes, removed supernatant | ||
| + | Added PEG 8000 and let stand in 4 C overnight. W3110 - 2.4 g BL21 - 2.5 g | ||
| + | |||
| + | ===5/16/13=== | ||
| + | |||
| + | DL 05/16 | ||
| + | |||
| + | Centrifuged at 8000 rpm (10,000 g) for 15 minutes. | ||
| + | Removed supernatant and added phage suspension buffer W3110 - 390 microL BL21 - 360 microL | ||
| + | Left overnight | ||
===5/17/13=== | ===5/17/13=== | ||
| + | |||
| + | AB 5/17/2013 | ||
| + | |||
| + | Centrifuged (4C) at 5500 rpm (5,000 g) for 10 min. | ||
| + | Added an equal volume of chloroform, let sit for 1 min, centrifuged (4C) at 5500 rpm (5,000g) for 15 min. | ||
| + | Saved the phage containing supernatant in eppendorf tubes, placed in the fridge until we decide what to do with them. | ||
| + | |||
| + | Over the weekend: come up with a community outreach program! | ||
| + | |||
| + | AC 05/17/2013 | ||
| + | |||
| + | We continued the purification of our phage. | ||
| + | We centrifuged both lysates at 5500 rpm (5,000 g) for 10 min at 4C | ||
| + | We next added an equal volume of chloroform to both tubes and let them sit for about a minute. Again we centrifuged the lysates at 5500 rpm for 15 min at 4C and removed the supernatant. | ||
===5/20/13=== | ===5/20/13=== | ||
| + | Today we tested to see if our phage that we purified were viable. To do this we ran a titer. We took 2x top agar and added LB to create a 1x top agar solution. We then added 4.5 mL top agar to .5 mL bacteria (W3110 and BL21) and spread that on the plate. We also performed a dilution series on our phage. We added 90 microL LB to 6 different tubes for each phage. Then we added 10 microL of each phage to the first tube and diluted it down to -5. We then spotted 2 microL of each tube on the plates (T4 on the W3110 and T7 on the BL21) and left them in the 37C incubator overnight. | ||
| + | |||
| + | Results: | ||
| + | |||
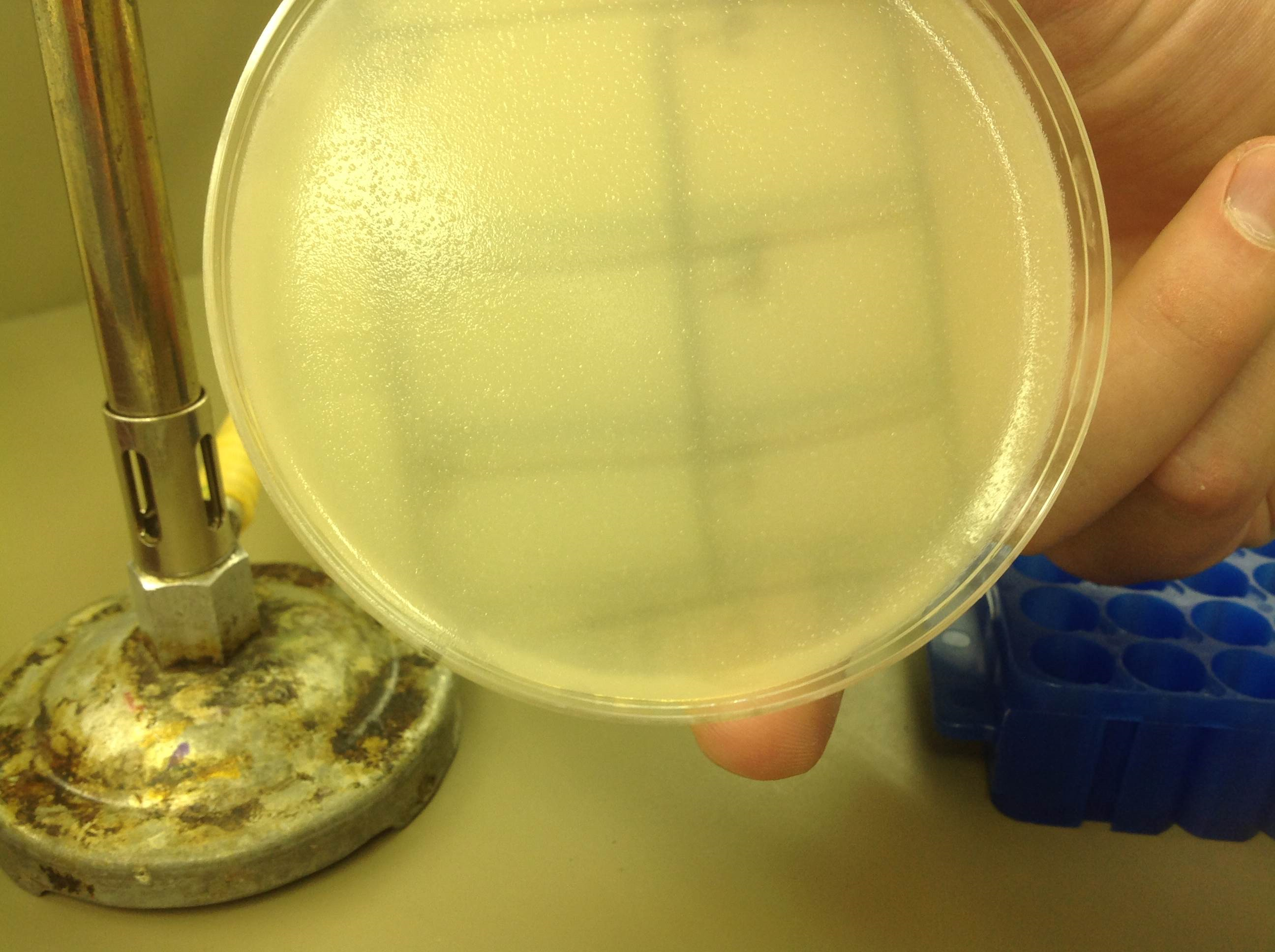
| + | :Our W3310 plate showed no phage plaques at all and showed signs of possible contamination. | ||
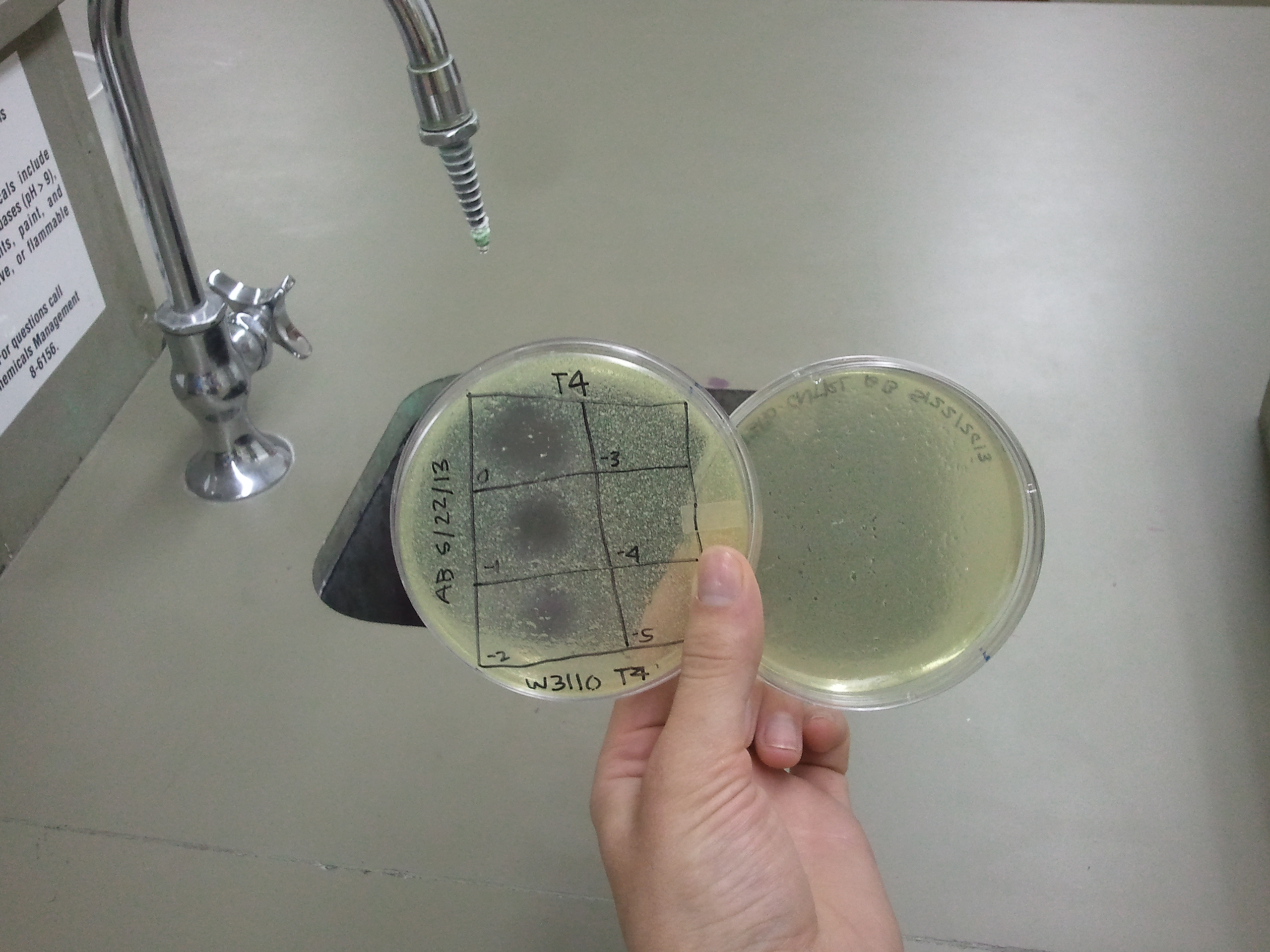
| + | :Our BL21 plate showed phage plaques, but they smeared so it was not possible to differentiate between the concentrations | ||
| + | |||
| + | [[File:W3110_5.22.13.png | thumb|none|alt=A no phage plaques were found on this sample.|w3110]] | ||
| + | [[File:BL21_5.22.13.png | thumb|none|alt=A phage plaques were found, but they had run.|BL21]] | ||
| + | |||
| + | AB AC DL | ||
===5/22/13=== | ===5/22/13=== | ||
| + | |||
| + | We created a new stock (25 mL) BL 21 bacteria. | ||
| + | |||
| + | We did a repeat of the phage titer of T4 phage from the isolated phage because of contamination on the last titer. We used bacteria from a glycerol stock and placed two loopfuls of bacteria into 25 mL LB broth. We waited an hour and used .5 mL of the bacteria to plate the titer and .5 for the control plate. The rest we incubated at 37 C.We followed the titer procedure from 5/20/13. | ||
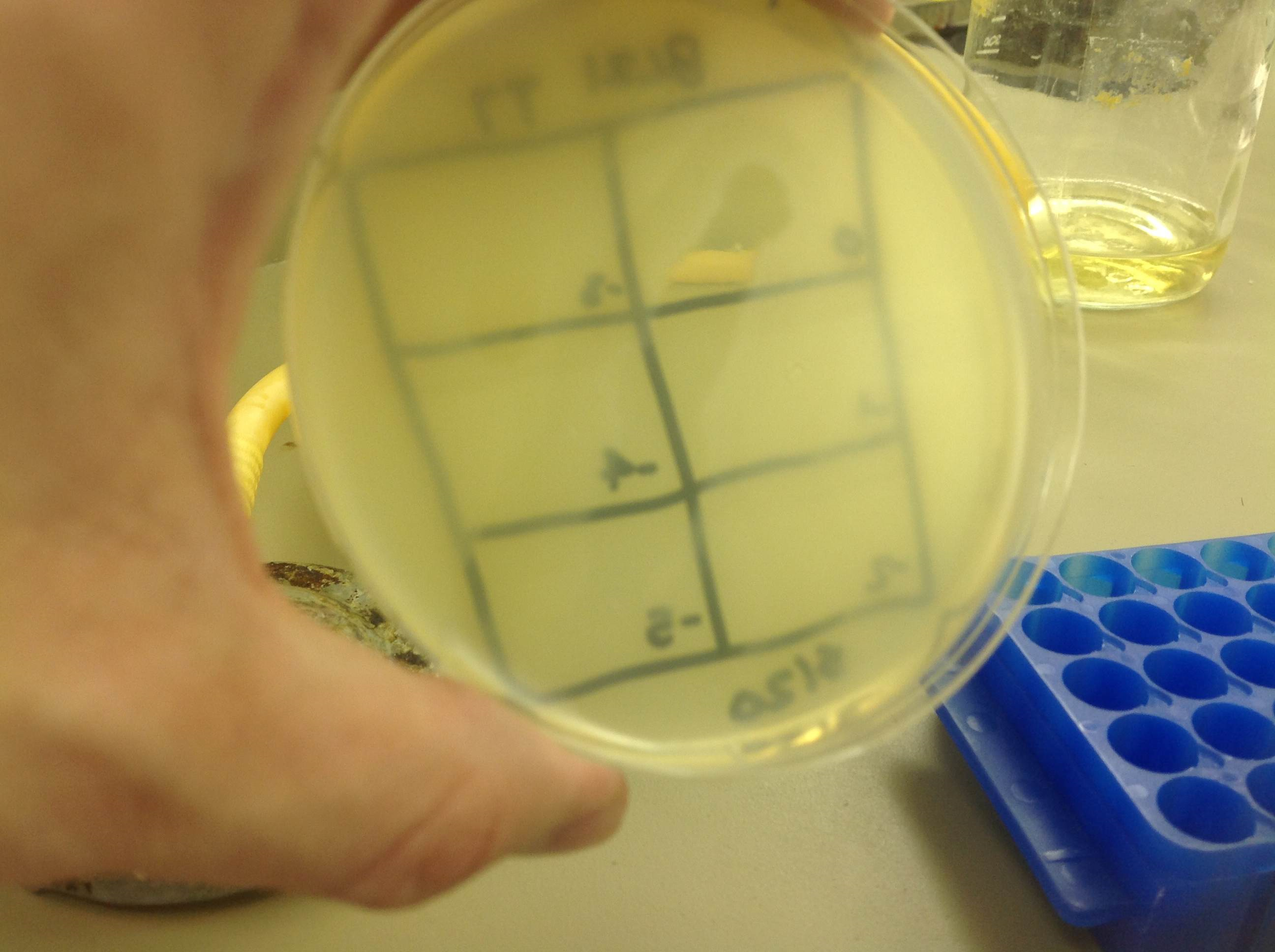
| + | Results: There was again contamination on both plates, the control and spot test plates, but plaques were still visible, proving the presence of viable phage after purification. | ||
| + | |||
| + | [[File:w3110_titer_5.22.13.png | thumb|none|alt=A phage plaques were found but with contamination.|w3110]] | ||
| + | |||
| + | AC AB DL | ||
===5/24/13=== | ===5/24/13=== | ||
| + | |||
| + | Today we put together a Cesium Chloride gradient to further purify our phage. | ||
| + | |||
| + | We added 1.23 g of CsCl to 6 ml of phage suspension buffer to create a 1.3 g/ml density gradient | ||
| + | |||
| + | We added 2.049 g of CsCl to 6 ml of phage suspension buffer to create a 1.5 g/ml density gradient | ||
| + | |||
| + | We added 2.46 g of CsCl to 6 ml of phage suspension buffer to create a 1.6 g/ml density gradient | ||
| + | |||
| + | We then layered 2 tubes with 2 mL 1.6 g/mL, 3 mL of 1.5 g/mL, and then 3 mL of 1.3 g/mL | ||
| + | |||
| + | We added all of our suspended phage of both t4 and t7 into the two separate tubes and then filled the tubes up all the way to about 3-5 mm from the top with phage suspension buffer. | ||
| + | |||
| + | We then placed the two tubes into the ultra-centrifuge and spun at 26500 rpms to achieve 100,000 g for 2.5 hours | ||
| + | |||
| + | We also created 50 mL cultures of BL21 and w3110 by adding 2 mL bacteria to 50 mL LB broth and incubating at 37 C overnight. | ||
| + | |||
| + | AC AB DL | ||
===5/27/13=== | ===5/27/13=== | ||
Latest revision as of 22:06, 24 May 2013
Contents |
March
3/15/13
Today we began researching for a procedure to begin purifying phage. We have found that T7 can self assemble with scaffolding proteins without forming procapsids, and that T4 has only been know to form procapsids. We may not have to worry about T4 if the other group isn't using it.
Important findings - Phage have been purified before Phage capsids can self assemble Phage (amber strain) can have their genes knocked out to make a hollow phage
3/18/13
Priorities List: Find out how to put drugs into the capsid Possibly contact F.W. Studier for his amber t7 phage strain Next class we plan on growing up phage so that we will have a decent amount of phage to work with. We have several procedures that we plan on testing so that we can see if we can purify the protein capsid. We hope to be proceeding with these as soon as we have phage that we can use. Another issue that we need to consider is how to get drugs into the capsid. We need to be able to test and see if we can actually fill our empty capsids with a material. We have several procedures found in other papers that could possibly help us with this.
3/20/13
AC
I spent time trying to find a good procedure to propagate phage in a liquid medium. I finally found one that I think will work well for us.
Today we learned a procedure for how to count phage. We performed a phage titer on the T4 phage to see if we have a high enough concentration to work with. This is the general procedure: Phage titer: reported in Pfu/mL (Pfu stands for plaque forming unit) mix E. coli (500 microL) with 50 microL of phage lysate incubate for 20 minutes mix with 4 mL top agar and then plate holes will appear in the agar that are called plaques
How we followed it: We filled five test tubes full of 90 microliters of Liquid Broth each. We added 10 microliters of our desired phage to the first test tube and then mixed We took 10 microliters of the first tubes mixed solution and added it to tube 2, and followed the same procedure for each test tube down the line to tube five. We then labeled 6 culture tubes 0 to -5. In the first culture tube, we added 20 microL phage to .5 mL bacteria. In tubes -1 to -5 we took 20 microL from eppendorfs and added to .5 mL bacteria. We then allowed a 20 minute waiting period for the virus to infect the E. coli. Then, 5 mL top agar was added to each culture tube. Each tube was then plated and incubated at 37 degrees C.
Amber - T2 Arick - T5 Darren - T3
DL We ran into several problems while doing the titer. After we had completed the titer, we found out that the pipet tips we had used were contaminated. When preparing the top agar, we had to melt it in the microwave which caused it to boil over. This could have caused some contamination. While filling my -5 plate with top agar, there was only enough to put in 4mL of agar instead of the 5mL that was called for in our procedure.
RESULTS: 3/22 None of the plates had any phage. There was just a lawn of bacteria growing. This could either be because of the problems mentioned above or because the source of T3 was bad. Seeing as nobody else was able to grow any phage we believe that the source was old.
3/22/13
AC 3/22
We had several sources of error in our procedure. First off there was contamination that affected our results. The micropipette tips that we used had not been autoclaved. This caused contamination to several plates. We also microwaved the augar instead of using a heat bath. This caused there to be tons of condensation in the plates and the augar to crack. We didn’t have any plates that had phage present.
Results: Despite the sources of error, it was pretty clear that the phage did not react with the bacteria. This was either because the phage was not viable, or because of the contamination.
AB 3/22/2013
None of the plates showed phage. A big problem that we had was contamination, but our agar was also crystal looking and cracked in several places. sources of contamination were accidental use of unautoclaved pipettte tips, and the agar was microwaved and exploded, causing water condensed in the microwave to fall into the agar. Results: No phage, lots of contamination. We may need a new source of phage, or just better lab technique.
Next step: find procedures for purifying different phage. Become experts on phage structure. possibly help with designing point mutations.
- osmotic procedure
- procedure from Dr. Grose
Phage structure: T7 has proteins 10 A and 10 B for capsid, and an assembly protein T4 has SOC and HOC proteins
DL 3/22
We are currently waiting for our phage to come in so that we can begin running tests to purify the capsid. We have several procedures in mind. We need to prepare the reagents so that when the virus comes in we can immediately begin attempting to purify it. Most of the procedures we have found apply specifically to T7 but we will also try them on T4. We will continue to look for other procedures that may apply specifically to T4.
3/25/13
AB 3/25/2013
Come up with a list of needed reagents for phage purification, so that when phage arrive we can begin testing procedure effectiveness for phage purification. Our findings may also be useful for the cholera group, since they also need phage to disrupt biofilms/kill cholera. List of materials for Phage Purification Procedure from Dr. Grose
1. Phage suspension buffer also called TM buffer (Tris-Mg2+ Buffer) 10 mM Tris–HCl (pH 7.2–7.5), 100 mM NaCl, 10 mM MgCl2. Addition of 1–10 mM CaCl2 in the suspension buffer may be required for the stability of some phages.
2. DNase I and RNase A from Bovine Pancreas (Roche or Cal- biochem). Stock solutions 1 mg/mL are stored at −20 ◦C.
3. Chloroform.
4. Sodium chloride powder: NaCl ≥ 99. 5 % for molecular biology.
5. Polyethylene glycol powder: PEG 6,000 (MW 5,000–7,000 g/mol) for molecular biology and biochemicalpurposes.
6. Cesium chloride: CsCl ≥ 99. 9 % for density gradient purification.
7. Ultracentrifuge equipments: Beckman L8-55M or equiva- lent.
8. Swinging-bucket rotors (Beckman): SW41 or SW28 and SW50 or SW65.
9. Centrifuge tubes (Beckman): thinwall or thickwall open-top polyallomer tubes:
- 13.2 mL, 14 mm × 89 mm for the SW41 rotor.
- 38.5 mL, 25 mm × 89 mm for the SW28 rotor.
- 5.0 mL, 13 mm × 51 mm for the SW50 or SW65 rotors.
10. Syringes and 18–22 gauge hypodermic needles.
11. Dialysis tubing: Spectra/Por molecular-porous membranetubing, MWCO 12–14,000.
12. Refractometer (optional).
Another Procedure to use: https://cpt.tamu.edu/wp-content/uploads/2011/12/CsCl-phage-prep-08-17-2011.pdf I think it uses the same materials as above, but the procedure is really easy to follow. Procedure for the self assembly of T13 phage - not sure if we'll need this https://cpt.tamu.edu/wp-content/uploads/2011/12/CsCl-phage-prep-08-17-2011.pdf Next, need to figure out what we are going to do with our purified phage - cleave tails? mutate for cholera group?
AC 03/25/2013
Plan of attack for the week: We will be coming up with a list of reagents needed for the purification of proteins. We are still waiting for purified phage to start propagating them and purifying them. In the meantime we are still reading papers on capsid structure and design as well as applications of capsids in nanotechnology. We have found some interesting articles on capsid batteries and drug epitopes. Articles are on the learningsuite website.
DL 3/25
What we need to do this week: Get a list of reagents, make sure we have the procedures down.
Osmotic shock materials: phage, 3M Na2SO4, 2.8M MgSO4, DNAse, centrifuge that can control temperature at 10 degrees C, saline
1. 2.9 ml. of the above phage + 6 ml. 3M Na2SO4 for 2 min., then + 140 ml. cold water rapidly with agitation Residual infectivity by plaque count was 5 X 108/ml.
2. Number 1 + 0.15 ml. saturated MgSO4 (2.8M) + 0.15 mg. DNAse left at 5°C. overnight
3. Number 2 centrifuged at 3500 for one half hr.; supernatant
4. Number 3 centrifuged at 100,000 X g for 1 hr. in a Spinco refrigerated centrifuge at 10°C.
5. Supernatant from number 4
6. Residue from number 4 dissolved in cold saline
7. Number 6 centrifuged at 2,000 for 15 min.; supenatant
8. Number 7 centrifuged at 18,000 × g in a Servall SS-2 for 1 hr.
9. Supernatant from number 8.
10. Residue from number 8 dissolved in saline
3/27/13
AC 3/27/2013
Found a great article on the structure and proteins of bacteriophage T4 http://www.farisaka.bio.titech.ac.jp/text/CMLS-Leiman-20031.pdf The paper goes into detail on the morphogenesis of the capsid head, tail, and LTF. I will be using this paper for the bulk of my presentation on Monday. The main proteins of my focus will be:
- GP-23
- 160 hexamers
- GP-24
- 11 pentamers
- GP-20
- dodecamer portal vertex
- GP-SOC & GP-HOC
- increased vitality
- T = 13 & Q = 21
DL 3/27
Capsid formation can follow several different pathways. This first is which proteins come together by single bonds, gradually forming the different parts that make up the capsid. In the second pathway, different parts are formed and then come together making multiple bonds at one time.
T4 structure http://link.springer.com/content/pdf/10.1007%2Fs00018-003-3072-1 shell has icosohedral ends and a cylindrical equatorial midsection that has a unique portal vertex where the tail attaches
head has gp hoc and gp soc attached to the outside of it gp soc helps maintain head integrity in extreme environments but neither gp hoc or gp soc are required head and midsection are formed by the gene product (gp)23
AB 3/27/2013
How they use T7 in medicine - kit from Novagen - http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3499470/ Bacteriophage T7 has six tail fibers that attatch to E.coli. These fibers are homotrimers (composed of three identical units of polypeptide) of gene 17. These kinked fibers attach to the LPS on E.coli.
Replication: The bacteriophage T7 replisome is an ideal model system for studying replication dynamics because it recapitulates the key features of more complicated systems, yet can be reconstituted in vitro with only four proteins. DNA synthesis is carried out by a stable, one-to-one complex of gene 5 protein (gp5) and thioredoxin, a processivity factor produced by the E. coli host. Gene 4 protein (gp4) contains both helicase and primase domains whereas gene 2.5 protein (gp2.5) is the single-stranded DNA binding protein (15).
Head made of 415 copies of 10B protein
Large-scale Amplification and Purification of T7 Phage Nanoparticles
The T7-p66, T7-p66x2 and T7-wt nanoparticles were propagated in the log phase culture of E. coli BL21 (OD600~0.8) grown in M9-LB broth (LB broth supplemented with 50 ml 20X M9 salts, 20 ml 20% glucose and 1 ml of 1 M MgSO4 per liter) at a multiplicity of infection (MOI) of 0.001 and incubated at 37°C until complete lysis of the culture (3–6 hours). Thirty minutes before removing the culture from the shaker, DNAse I and RNAse A (Roche, Germany) were added to degrade released bacterial nucleic acids. T7 phage nanoparticles were precipitated from the culture supernatant by addition of 1 M NaCl and 10% polyethylene glycol (PEG 6000, Merck) followed by overnight incubation at 4°C. The T7 phage pellet was resuspended in Tris-NaCl buffer (PH 8) and PEG and cell debris was removed by centrifugation at 10,000 rpm for 10 min. To remove residual PEG and debris, an equal volume of chloroform was added, gently inverted and the top aqueous phase was harvested after low-speed centrifugation at 4°C. The purified T7 nanoparticles were sterilized using a pyrogen-free 0.2 µm pore-size cellulose acetate filter (Millipore) and stored at 4°C until further analysis.
Removal of Bacterial Endotoxin from T7 Phage Nanoparticles
The bacterial endotoxin (LPS) concentration in all T7 nanoparticle preparations was determined in triplicate using a sensitive colorimetric Limulus Amebocyte Lysate (LAL) QCL-1000® kit (Lonza, USA) according to the manufacturer's instructions. LPS was removed from T7 phage nanoparticles based on a method for removal of endotoxin from protein solutions by phase separation using Triton X-114 as described by Aida et al. [22] and modified by Hashemi et al. (manuscript submitted for publication).
Genes of T7: http://www.uniprot.org/uniprot/?query=organism%3a10760+keyword%3a1185&offset=25 Thus phage T7 has an icosahedral shape with an edge of 37.7±0.5nm, a volume of (120±10)x103nm3, and a small tail that is 6-7% of the head volume. (STRUCTURE OF BACTERIOPHAGE T7 Small-AngleX-rayandNeutronScatteringStudy)
How T7 infects: http://www.sciencemag.org/content/339/6119/576.full.pdf?sid=178e4a68-d44f-4f10-b403-1ef7f893efa8 (excellent pictures for structure)
3/29/13
DL 3/29 We prepared our presentation on a power point for this upcoming Monday. We will be presenting on the background and structure of T7 and T4
AC 3/29
We put together our powerpoint presentation for Monday. I took all my information from http://www.farisaka.bio.titech.ac.jp/text/CMLS-Leiman-20031.pdf
April
4/3/13
AB 4/3/2013
FINAL PRESENTATION- attend 1 science talk. 11 or 4 on thursdays. Write a paragraph on what made it a good talk. GROUP GRADE: Science talk (15 min): 1-Background (5-10 min) - what's your hypothesis? What question are you answering and why? 2-Method/Results (5-10 min) - usually a cycle 3-Conclusions - should be clear. Be repetitive. Due 4/15/2013
Today we will be setting up an overnight culture of E. coli, to grow for use with phage amplification. We will take a 500 flask; with 50 mL LB broth, and place a culture form the E.coli culture plate to grow overnight. We will create a glycerol stock of the E.coli culture to save in the -80 C freezer. Liquid culture 4/2/2013 We put a colony from our W3110 plate of E.coli into 1mL LB broth and placed in the 37 C incubator overnight. We did this with 5 colonies. Results: the control tube of broth showed no contamination. We also put 500microL of 40% glycerol into cryovials for use tomorrow to prepare freezer sotcks.
DL 4/3
Final project presentation - attend a science talk before and write a paragraph about what made it good/bad what makes a good presentation
- background - hypothesis (why?/what?) 5-10 minutes
- method/results 5-10 minutes
- conclusions
“tell them what you are going to tell them, tell them, then tell them what you just told them” due april 15th
Our T4 phage arrived today.
We also prepared a glycerol stock to save in the -80 C freezer.
Added .5 mL glycerol of 40% glycerol and .5 mL liquid culture from overnights. Put in the -80 C freezer.
AC 4/3
Final Presentation Good Presentation: Background that is gripping and compelling that will draw them into the presentation. (5-10 minutes) Methods / Results Conclusions Be repetitive - Tell them what you’re going to tell them, tell them, and tell them again.
Our T4 phage arrived today, so we will be working on making the overnight E. Coli cultures for work on Friday. We took 50 mL of liquid broth and added some W3310 E Coli from our culture plate and put it in the 37 C incubator overnight
We also put 1 mL of liquid broth in 6 tubes and added a culture of W3310 E Coli to 5 tubes. The control had no contamination the next day.
4/4/13
4/4/2013 AB Glycerol Stocks of W3110 We took the .5mL glycerol in cryovials and added .5mL liquid culture form the overnights prepared on 4/3/2013. We vortex the tubes for about 10s each. Next, we labeled the tubes w3110 E.coli 4/3/2013 and placed them in the -80C freezer.
04/04/13 AC
We made glycerol stocks W3110 E. Coli We put .5mL glycerol in cryovials and .5mL liquid culture from the overnights prepared yesterday. We vortexed each tube for 10 seconds and then we stored the tubes in the -80°C freezer in Dr. Grose’s lab.
4/5/13
04/05/13 AC
We did spot tests of phage on E. Coli BL21 We plated together .5mL of BL21 with 1XLBTA. We separated the plates into different segments and then spot tested the following phages. 14 T3 13 T5 10 T2 20OX171 40ØX174 The plates were then put in a 37°C incubator
4/5/2013 AB
Spot Tests of phage on BL21
We plated .5mL BL21 with 1XLBTA on a plate. Then, we took 5microL of each phage and put them in seperate quadrants on the plate. The phage we used were:
14 T3
13 T5
10T2
20OX174
40ØX174
We then put our 37°C incubator overnight
Results: there were no plaques on any of the plates. There was no contamination in the control quadrant. An uniform bacterial lawn formed with no phage infection.
DL 4/5
We did spot tests of phage on BL21. Plated .5mL BL21 m w/ x1 LBTA. Then took 5 microL of phage and put on seperate quadrants of the plates. The phage we used are: 14 T3 13 T5 10T2 20OX174 40ØX174 We placed these in the 37 C incubator overnight.
4/8/13
DL 4/8
We performed a phage titer spot test on two bacteria. We used the BL21 and W3110 strains of E. coli. We first put 100 microL of broth into epindorf tubes. We then took 10 microL of 1L, 10L, 40T4, T4DOS, and T4 infected phage and placed it in seperate tubes labeled 0. We performed a dilution series adding 10 microL of phage to the tube -1 and continued down to -5. Next we added 4.5 mL of 1x top agar to .5mL of both strains of bacteria and plated it on LB plates. We had previously divided the plates into six sections with the labels 0, -1, -2, -3, -4, and -5. We took the phage from each concentration and spotted it on the plates. We allowed the plates to sit and let the phage soak in. We then placed the plates in the 37 C overnight.
Results: 04/10 40T4 did not grow on W3110. All the other plates formed plaques. All of the plates experienced some sort of running. Controls showed no contamination and grew a lawn of bacteria.
AC 04/08
We performed a phage titer test to determine which bacteria would be most viable for our phage propagation. We used BL21 and W3110 strains of E. Coli. We placed 100 µL of broth into 5 epindorf tubes. We used as phage 10 µL of 1L, 10L, 40T4, T4DOS, and T4 infected phage and placed them each in one of tubes labeled -1. We performed a dilution series taking out 10 µL each time and placing it into the next tube, 5 times. the total volume in the last tube was 110 µL. We added 4.5 mL of 1X top agar to .5 mL of broth and plated it on LB plates. We spotted each concentration on the plates and incubated it overnight at 37°C.
Results from 04/08:
Phage 10L w/ w3110 had large scale infection every concentration
Phage 10L w/ BL21 had infection in very large concentrations
Phage 1L w/ w3110 had infection in very large concentrations
Phage 1L w/ BL21 had infection in very large concentrations
Phage T4 DOS w/ w3110 had infection in very large concentrations
Phage T4 DOS w/ BL21 had infection in very large concentrations
Phage 40 T4 w/ w311o had no phage infection
Phage 40 T4 w/ BL21 had large scale infection at every concentration
Phage T4 inf w/ w311o had large scale infection at every concentration
Phage T4 inf w/ BL21 had large scale infection at every concentration
We had a significant amount of running that occurred on almost every plate, so the results were a little difficult to read. We concluded that the phage is a high enough concentration that we would have to do another phage titer to a smaller dilution in order to determine actual concentration. The controls were all a lawn of bacteria.
Results: 04/08 no plaques or phage infection. bacterial lawn grew. no contamination noted.
4/10/13
AC 04/10
We performed titers of T4 infected phage from off of two separate E. Coli strands, BL21 and W3110. We added .1mL of the liquid culture to 1 mL of both strands of bacteria. After allowing infection to occur overnight, we centrifuged the tubes to separate the bacteria from the phage. We separated the supernatant into a new eppendorf tube, and used 10 µL as the 0 concentration. We then performed a phage titer using five tubes of 90 µL LB each, adding 10 µL from each one to the next. Using a micropipette we spotted 2µL of each dilution onto both 4.5 ml 1x plates and .5 mL W3110 plates. To avoid smearing, we allowed the plates to sit for over an hour and then we incubated them at 37 C overnight.
Results: The concentrations we used are still way too strong. We will have to dilute the concentration even further if we want to see anything more than cleared plaques.
DL 04/10
We performed another titer of T4 infected phage. T4 phage had been plated on BL21 and W3110 and pulled off. We took .1 mL of this and added it to 1 mL bacteria of both cultures. After allowing to grow overnight, we centrifuged to pellet the bacteria and took off the supernatant of the phage. We then did a dilution series using the supernatant as 0 concentration. We diluted it up to -5 by adding 10 microL to 90 microL of LB. We then spotted 2 microL from each tube on 4.5 mL x1 LBTA and .5 mL W3110. Allowed them to sit for an hour and a half and placed the plates in the 37C overnight.
AB 4/10/2013
Today we did a titer of the T4 infected phage that was pulled off a plate of T4 phage on BL21 and W3110. We took .1mL of this liquid culture and added it to 1 mL bacteria of both cultures. We allowed the phage to infect overnight, then centrifuged to form a pellet. We decanted the supernatant into a new eppendorf tube and used 10 microL as our 0 conc. in the titer assay. We performed the titer using 5 tubes of 90microL LB, transferring 10microL from each tube to the next. We spotted 2microL from each tube on 4.5 mL 1x TA and .5 mL W3110. We allowed them to sit for an hour and a half before placing the plates in the 37C incubator overnight. Results: WE had plaques on both plates, but we have yet to achieve what appears to be a dilution - we get only a cleared plaque on every concentration. The control was clear of contamination.
4/12/13
AB 4/12/2013 Notes on Cholera presentation: Current testing method to identify cholera is long and expensive. Begin by explaning that you can sense both presence and non presence of cholrea via RFP and GFP - GFP equal cholera present/high cell density, and RFP = low cd. Does this give false negatives? -Know when to skip useless information!!!
DL 04/12
We watched presentations by the cholera group. Their main idea is going to have the bacteria fluoresce different colors based on whether or not cholera is present.
AC 04/12
We watched the cholera presentation today. It looks like they will be trying to use fluorescent proteins to detect different densities of cholera.
4/15/13
AB 4/15/2013 We watched the presentations of the large and small phage groups, and presented our project and ideas.
DL 04/15
We presented our progress so far as the phage group. Two of the main questions that arose was how we are going to know if we have ghost capsids and how we are going to know if they are viable. We need to research this so that we know how to test for this.
AC 04/15
We presented our project today. There were a few things that we needed to do better. For one, we need to present better background and show how it ties into our research, specifically the need for a capsid library. I feel like we could have been a bit more engaging and better rehearsed as well.
We will need to find a way to test the capsids for viability, and somehow characterize them.
May
5/1/13
DL 05/01
Look at Utah State's wiki for last years project to get a good idea of a cool website. Talk to Dr. Grose about how pure our sample needs to be. We prepared a document with a step-by-step plan of what our goals were for the rest of the semester. We also read through the purification procedure that we will be doing Monday to ensure that we understand everything and have all the materials.
AB 5/1/2013 Today we are going to create a plan to follow throughout the semester. We also talked with Jordan about reagents for performing phage purification on Monday. We also reviewed the methods for our procedure.
-Plan to make an excel sheet that will calculate the needed volumes of reagents for the volume of lysate used.
-Plan how many days it will take to complete the procedure - plan in breaks and time Questions to ask Dr. Grose - what is less expensive/better - Freeze thaw or use lithium chloride
- Answer: Try both
-Best method for determining phage purification
-Step 3 on 3.3.1.1
-CsCl gradient - we may need someone with experience to help
-should we plan on using CsCl? Or just plan on PEG? (time to set up gradient)
-Step 7 on 3.2.1 (dialysis??)
AC 5/1/2013
Today we created a detailed tentative plan to follow for the rest of the semester. https://docs.google.com/document/d/1ffqtGWjGOqEuOgNd46yK38lzkzMVdoCgXPcxMcWfCcc/edit?usp=sharing We went through the protein purification procedure to figure out the times that each step would take. https://docs.google.com/document/d/1W56-4bIIKsWBDtxsY2VgwV1q5BTo2nIXmrOLuqA0U9A/edit?usp=sharing
5/3/13
AC 5/3/2013
We created an excel sheet to help us easily calculate the amount of reagents we need for the protein purification protocal
DL 05/03
We created an excell sheet to calculate the amounts of each reagent for the experiment we will be running on Monday. This will allow for easy calculations each time we run the experiment.
5/6/13
AC 05/06/13
We created more bacteria stock of W1130 and BL21
For BL21 we put 1 mL of BL21 into 24 mL of LB and incubating overnight at 37 C
For W3110 we put 1 mL of W3110 into 24 mL of LB and incubating overnight at 37 C
We also centrifuged 5 mL of t7 lysate in 5 1 mL ependorf tubes and separated the phage supernatant into 5 1 mL ependorf tubes and stored it into the fridge.
We are still waiting on supplies for propagation
Results: Our W3310 didn’t grow in the liquid culture we will have to create a new one.
AB 5/6/2013
We learned that the T7 Phage requires 24-48 hours to infect. We infected 24 mL LB broth with 1 mL Bacteria (W3110 and BL21 in separate Erlenmeyer flasks) and incubated overnight, W3110 at 30 C and BL21 at 37 C. Results: BL21 grew well, while W3110 had no growth. We also took a 5 mL bacterial lysate of T7 and BL21 and centrifuged for 5 min at 3050 rpm in 2 mL eppendorf tubes to preserve the phage. We moved the supernatant into clean eppendorfs and placed in the fridge for future use..
DL 05/06
Our order of PEG 6000 and CsCl hadn’t arrived yet so we weren’t able to perform the first experiment. We prepared more bacteria stock of W1130 and BL21. For each we placed 1 mL of bacteria in 24 mL of LB and incubated at 37 C. We also separated T7 phage from bacterial lysate. We had 5 mL of bacterial lysate that we separated into five 1 mL ependorf tubes and centrifuged for 5 minutes. We then took the supernatant and stored it in five 1 mL ependorf tubes.
5/8/13
AC 05/08/13
Most of our reagents came except for our DNAse hopefully it will come by Friday. We created more stock of both one BL21 and W3110.
We added 24 mL of LB to two erlenmeyer flasks. We added 1 mL of BL21 to one flask and 2 mL of W3310 to the other. The W3310 was then incubated for an hour at 30 C and the BL21 was incubated for an hour at 37 C
AB 5/8/2013
Today we made a new W3110 culture using 24 mL broth and 1 mL W3110 from liquid culture. We placed it in the 30 C incubator overnight. Results: this culture grew well. We also infected 24 mL LB broth with 1 mL BL21 and placed it in the 37 C incubator for 1 hour. We then infected with 100 microL T7 phage and placed back in the 37 C incubator for 48 hours. Finally, we crntifuged down 6 mL of bacterial lysate and saved the supernatant containing T4 phage in the fridge.
DL 05/08
We are still missing our DNAse to run the purification experiment. If it doesn't come by Friday we will probably go around asking professors if they have any. Today we prepared a W3110 culture and a culture of phage. For the W3110, we put 24 mL of broth in a flask and added 2 mL of W3110 from a liquid culture. We then put it in the 37 C incubator. For the phage culture, we put 24 mL of broth in a flask and added 1 mL BL21. Then we put it in the 37 C incubator for 1 hour. Then we infected it with 100 microL phage and put it back in the 37 C incubator for 48 hours.
5/10/13
AC 05/10/13
We are still waiting on some DNase to come in. We did infect 24mL of Broth with 1 mL w3110 and placed it into the 37 C incubator.
We emailed Dr. McCleary to see if he could train us on ultracentrifuge use. We also will have to order new centrifuge tubes....
AB 5/10/2013
Today we infected 24 mL broth with 1 mL w3110 and placed it in the 37 C incubator. We also emailed Dr. McCleary on ultracentrifuge use, and we organized the materials for our procedure. Assignment #2 is due on Monday. Write up about where we are at in our research.
DL 05/10
Today we grew W3110 by infecting 24 mL of broth with 1 mL of W3110. We put it in the 37 C incubator. It looks like we may have to order special tubes in order to use the centrifuge. We emailed Dr. McCleary about what tubes we should get and how we use the centrifuge.
5/13/13
AC 5/13/2013
Thankfully we don’t have to use the ultracentrifuge to achieve 10,000 g. We also won’t need to order any special centrifuge tubes, so we should be able to start experimentation on Wednesday.
We added 1 mL of w3110 and 1 mL of BL21 to 24 mL of LB each and placed them both into the 37C incubator. After an hour of incubation we infected the BL21 Ecoli with t7 phage and left to incubate until wednesday. We will infect the w3110 tomorrow with T4
AB 5/13/2013
Today we hunted down Dr. McCleary, and learned that we did not need to use the ultracentrifuge unti llater. we began new 25 mL solutions (24mL LB broth, 1 mL bacteria) and placed both w3110 and BL21 in the 37 C incubator. For BL21, we waited an hour and then infected with 100 microL phage. We then placed it back into the 37 C incubator for 48 hours. T4 will be infected tomorrow, due to its shorter incubation time.
DL 05/13
We found Dr. McCleary and he explained to us that we don’t need to use the special centrifuge until the later steps. We will be able to run the experiment at 10,000 g with a different one that we already have the tubes for. We should be running the purification on Wednesday. To prepare for it, we began growing BL21 and W3110. After an hour we infected BL21 with 100 microL T7 phage. Tomorrow, we will infect T4. We placed both back in the 37 C incubator.
5/14/13
AC 5/14/13
Today I came in and infected 24 mL of LB with 1 mL of w3310 and put in the 37C incubator overnight.
I also infected 25 mL of w3110 with 100 µL of t4 phage and left overnight in the 37C incubator overnight.
5/15/13
AC 5/15/13
We added 2.5 ml of LB to the 24 mL of t4 infected W3110 to make a final volume of 25 mL.
We added 92.5 µL of DNAse I and 12.5 µL of RNAse A into the 25mL of t4 infected w3110 Ecoli and the t7 infected BL21.
We then added 50 µL of chloroform to each lysate and let it incubate for 30 min at room temperature.
We then added .73 g of solid NaCl to both lysates and let it sit at 4 C for 1 hour.
AB 5/15/13
To make the W3110 with T4 a total volume of 25 mL, we added 2.5 mL to the 22.5 mL of lysate. Then we added 92.5 microL DNase 1 and 12.5 RNase A to each lysate (BL21, T7 and W3110, T4; 25 mL of each) and then added 50 microL Chloroform. We let this incubate for 30 min at room temperature. Next, we added .73 g solid NaCl and let the lysates sit at 4 C for 1 hour. (start 3:22, finish 4:22) Then, we centrifuged at 6500 rpm (7000xg) for 10 min. We removed the phage containing supernatant and put it in a clean tube. (24 mL W3110; 25mL BL21) After, we added PEG 8000 to a final concentration of 8-10% and let it stand for 1 hour. W3110 - 2.4g PEG BL21 - 2.5 g PEG
DL 05/15
Added 2.5 mL of LB to the 22.5 mL of T4 infected W3110 to make a final volume of 25 mL. Added 92.5 microL of DNase I and 12.5 microL of RNase A to both T4 and T7. Added 50 microL of chloroform and incubated at room temp for 20 min. Added .73 g solid NaCl and let sit at 4 C for 1 hour Centrifuged at 6500 rpm (7000 g) for 10 minutes, removed supernatant Added PEG 8000 and let stand in 4 C overnight. W3110 - 2.4 g BL21 - 2.5 g
5/16/13
DL 05/16
Centrifuged at 8000 rpm (10,000 g) for 15 minutes. Removed supernatant and added phage suspension buffer W3110 - 390 microL BL21 - 360 microL Left overnight
5/17/13
AB 5/17/2013
Centrifuged (4C) at 5500 rpm (5,000 g) for 10 min. Added an equal volume of chloroform, let sit for 1 min, centrifuged (4C) at 5500 rpm (5,000g) for 15 min. Saved the phage containing supernatant in eppendorf tubes, placed in the fridge until we decide what to do with them.
Over the weekend: come up with a community outreach program!
AC 05/17/2013
We continued the purification of our phage. We centrifuged both lysates at 5500 rpm (5,000 g) for 10 min at 4C We next added an equal volume of chloroform to both tubes and let them sit for about a minute. Again we centrifuged the lysates at 5500 rpm for 15 min at 4C and removed the supernatant.
5/20/13
Today we tested to see if our phage that we purified were viable. To do this we ran a titer. We took 2x top agar and added LB to create a 1x top agar solution. We then added 4.5 mL top agar to .5 mL bacteria (W3110 and BL21) and spread that on the plate. We also performed a dilution series on our phage. We added 90 microL LB to 6 different tubes for each phage. Then we added 10 microL of each phage to the first tube and diluted it down to -5. We then spotted 2 microL of each tube on the plates (T4 on the W3110 and T7 on the BL21) and left them in the 37C incubator overnight.
Results:
- Our W3310 plate showed no phage plaques at all and showed signs of possible contamination.
- Our BL21 plate showed phage plaques, but they smeared so it was not possible to differentiate between the concentrations
AB AC DL
5/22/13
We created a new stock (25 mL) BL 21 bacteria.
We did a repeat of the phage titer of T4 phage from the isolated phage because of contamination on the last titer. We used bacteria from a glycerol stock and placed two loopfuls of bacteria into 25 mL LB broth. We waited an hour and used .5 mL of the bacteria to plate the titer and .5 for the control plate. The rest we incubated at 37 C.We followed the titer procedure from 5/20/13. Results: There was again contamination on both plates, the control and spot test plates, but plaques were still visible, proving the presence of viable phage after purification.
AC AB DL
5/24/13
Today we put together a Cesium Chloride gradient to further purify our phage.
We added 1.23 g of CsCl to 6 ml of phage suspension buffer to create a 1.3 g/ml density gradient
We added 2.049 g of CsCl to 6 ml of phage suspension buffer to create a 1.5 g/ml density gradient
We added 2.46 g of CsCl to 6 ml of phage suspension buffer to create a 1.6 g/ml density gradient
We then layered 2 tubes with 2 mL 1.6 g/mL, 3 mL of 1.5 g/mL, and then 3 mL of 1.3 g/mL
We added all of our suspended phage of both t4 and t7 into the two separate tubes and then filled the tubes up all the way to about 3-5 mm from the top with phage suspension buffer.
We then placed the two tubes into the ultra-centrifuge and spun at 26500 rpms to achieve 100,000 g for 2.5 hours
We also created 50 mL cultures of BL21 and w3110 by adding 2 mL bacteria to 50 mL LB broth and incubating at 37 C overnight.
AC AB DL
5/27/13
5/29/13
5/31/13
June
6/3/13
6/5/13
6/7/13
6/10/13
6/12/13
6/14/13
6/17/13
6/19/13
6/21/13
6/24/13
6/26/13
6/28/13
July
7/1/13
7/3/13
7/5/13
7/8/13
7/10/13
7/12/13
7/15/13
7/17/13
7/19/13
7/22/13
7/24/13
7/26/13
7/29/13
7/31/13
August
8/2/13
8/5/13
8/7/13
8/9/13
8/12/13
8/14/13
8/16/13
8/19/13
8/21/13
8/23/13
8/26/13
8/28/13
8/30/13
September
9/2/13
9/4/13
9/6/13
9/9/13
9/11/13
9/13/13
9/16/13
9/18/13
9/20/13
9/23/13
9/25/13
9/27/13
9/30/13
 "
"