Team:BYU Provo/Notebook/SmallPhage/Winterexp/Period2/Exp/4.8 T7 phage viability assay 2
From 2013.igem.org
(Created page with "{{TeamBYUProvo}} <br> {| width="100%" | colspan="3" | <font color="#333399" size="5" font face="Calibri"> '''Small Phage May - June Notebook: Experiments'''</font> <br> <br> ...") |
|||
| (4 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
{| width="100%" | {| width="100%" | ||
| - | | colspan="3" | <font color="#333399" size="5" font face="Calibri"> '''Small Phage | + | | colspan="3" | <font color="#333399" size="5" font face="Calibri"> '''Small Phage March - April Notebook: Experiments'''</font> |
<br> | <br> | ||
| Line 14: | Line 14: | ||
<font color="#333399" size="3" font face="Calibri"> | <font color="#333399" size="3" font face="Calibri"> | ||
| - | : | + | <font size = "4"> |
| + | |||
| + | : <u> '''Small Phage''' </u> </font> | ||
: [[Team:BYU Provo/Notebook/SmallPhage/Winterexp|March-April]] | : [[Team:BYU Provo/Notebook/SmallPhage/Winterexp|March-April]] | ||
| Line 30: | Line 32: | ||
<font face="Calibri" size="3"> | <font face="Calibri" size="3"> | ||
| - | <font size="5"> ''' | + | <font size="5"> '''4.8 T7 Phage Viability Test 2''' </font> |
<br> | <br> | ||
| Line 38: | Line 40: | ||
- Test the viability (concentration if possible) of the T7 we got | - Test the viability (concentration if possible) of the T7 we got | ||
| - | - Become familiar with virus | + | - Become familiar with virus titering techniques |
- Identifying T7 mutant and T7 WT | - Identifying T7 mutant and T7 WT | ||
| Line 58: | Line 60: | ||
:i) 90μL of LB broth were added to added to each dilution vial, labeled 1-5. | :i) 90μL of LB broth were added to added to each dilution vial, labeled 1-5. | ||
| - | :ii) 10μL stock phage solution (T7*)was added to vial 1. | + | :ii) 10μL stock phage solution (T7*) was added to vial 1. |
:iii) Perform a serial dilution (1:10) with vial 1-5, decreasing phage concentration to 10% of the previous vial with each. | :iii) Perform a serial dilution (1:10) with vial 1-5, decreasing phage concentration to 10% of the previous vial with each. | ||
| Line 89: | Line 91: | ||
'''IV) Results''' | '''IV) Results''' | ||
| + | |||
1) Plates | 1) Plates | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | |+ | + | |+Spot Test Plates |
|- | |- | ||
|'''Label''' | |'''Label''' | ||
| Line 132: | Line 135: | ||
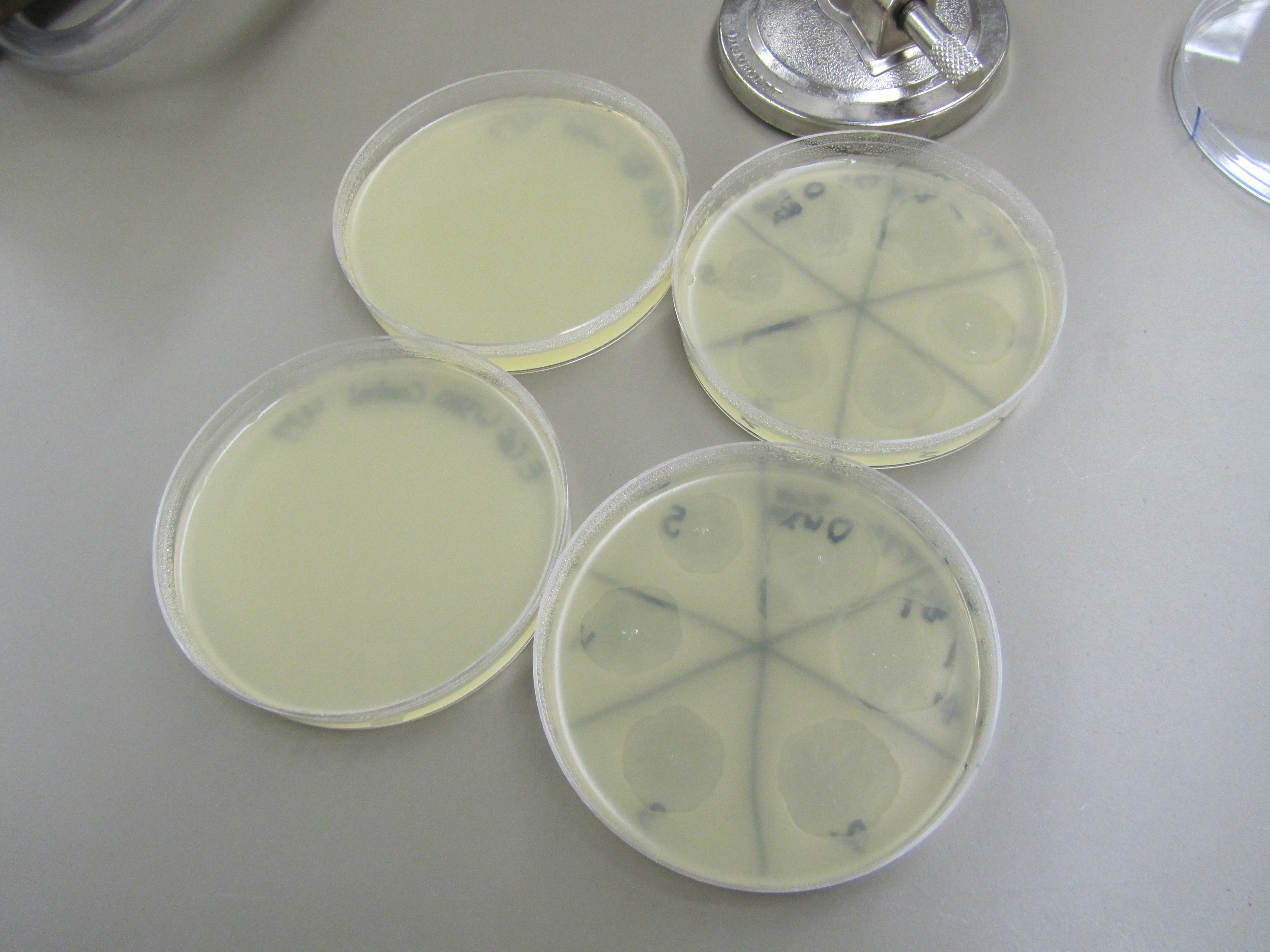
2) Spot test compared to control (top: BL21; bottom: W3110) | 2) Spot test compared to control (top: BL21; bottom: W3110) | ||
| + | |||
| + | [[File:4.8SpotTest.JPG|400px|center]] | ||
Circular plaques formed for each spot test. The plaques decreased in size as phage in LB becomes more diluted. No contamination seen. | Circular plaques formed for each spot test. The plaques decreased in size as phage in LB becomes more diluted. No contamination seen. | ||
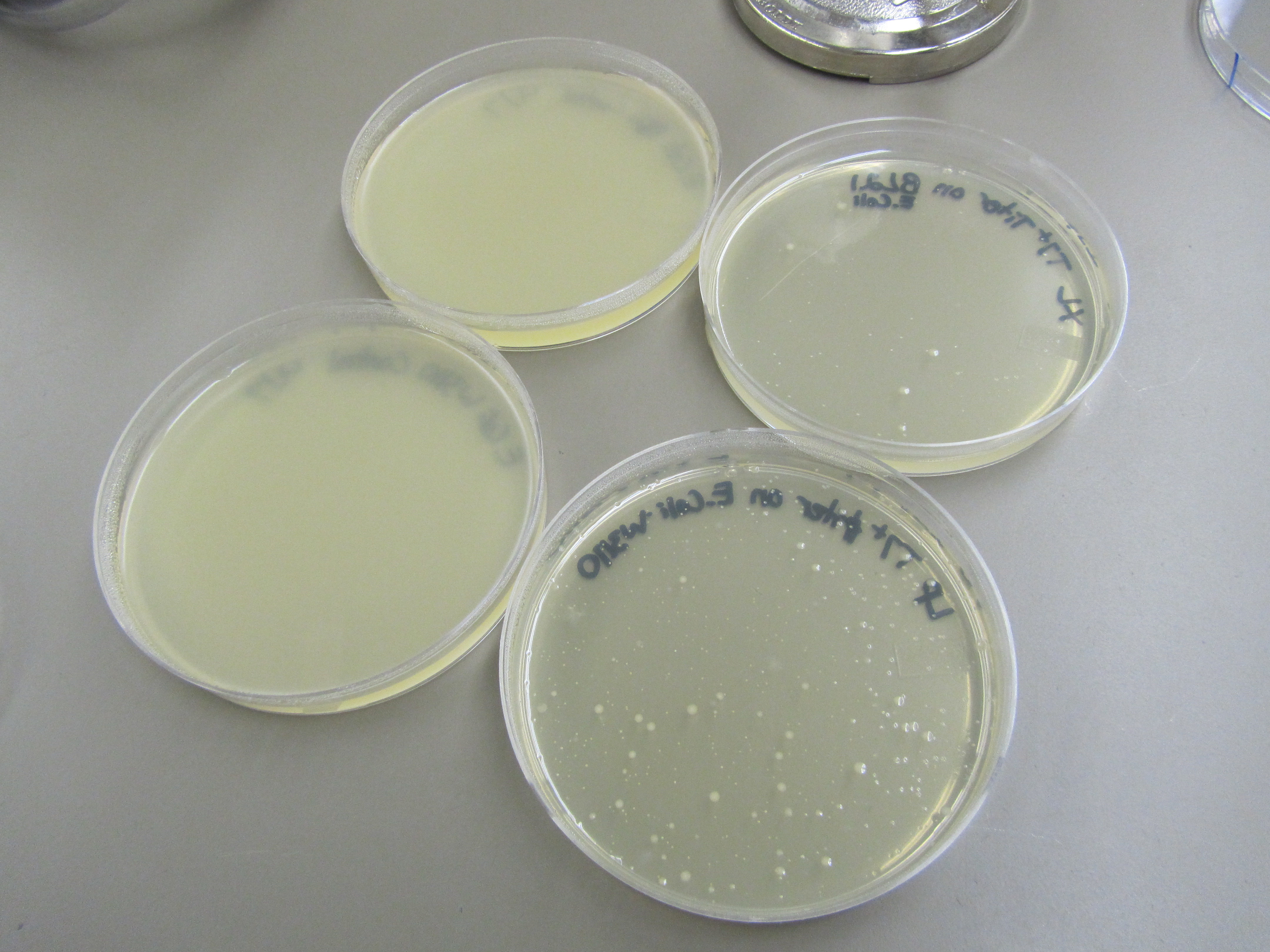
3) Titer test compared to control (top: BL21; bottom: W3110) | 3) Titer test compared to control (top: BL21; bottom: W3110) | ||
| + | |||
| + | [[File:4.8Titer.JPG|400px|center]] | ||
Phage is clearing up the entire plate. This indicates that the phage we used are at too high of a concentration. | Phage is clearing up the entire plate. This indicates that the phage we used are at too high of a concentration. | ||
Latest revision as of 13:28, 9 September 2013
| Small Phage March - April Notebook: Experiments
| ||||||||||||||||||||||||||||
|
|
4.8 T7 Phage Viability Test 2
I) Purpose - Test the viability (concentration if possible) of the T7 we got - Become familiar with virus titering techniques - Identifying T7 mutant and T7 WT II) Expected outcome - Should see multiple plaques forming on a lawn of E coli (confluent growth) - T7 WT should grow, even though T7 mutant didn’t (4.5 phage viability assay) III) Reagent record T7 phage *; E coli BL21; Agar: ×2 prepared by Jordan in 500mL glass bottle; LB: prepared by Jordan in big Erlenmeyer flask; overnight bacteria culture: set up on Sat 3/6 at 6pm IV) Actual procedures / observations 1) Dilution series of bacteriophage and its incubation with E coli
2) Perform spot test
30 Phage plaque size test
4) Check up on phage + bacteria viability in 24 hours. IV) Results 1) Plates
2) Spot test compared to control (top: BL21; bottom: W3110) Circular plaques formed for each spot test. The plaques decreased in size as phage in LB becomes more diluted. No contamination seen. 3) Titer test compared to control (top: BL21; bottom: W3110) Phage is clearing up the entire plate. This indicates that the phage we used are at too high of a concentration. V) Conclusions - T7* is the WT T7 phage. It is able to grow on E coli BL21. - Our stock phage liquid culture is at too high concentration to form a few individual plaques on a plate. It’s clearing up the entire area. VI) Proposed next step 1) Perform the same spot test with more diluted T7 solution. Look for where the plaques are barely forming.
| |||||||||||||||||||||||||||
 "
"