Template:Kyoto/Notebook/Sep 25
From 2013.igem.org
(Difference between revisions)
(→Colony PCR) |
(→Ligation) |
||
| (79 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Sep 25== | ==Sep 25== | ||
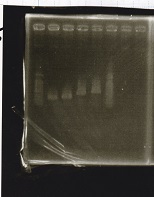
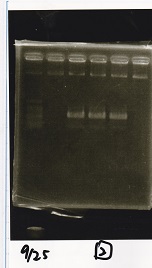
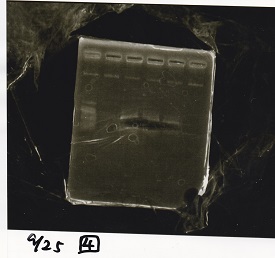
| - | + | ===Electrophoresis=== | |
| + | |||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||Pcon-antisense (1C3-66E-fwd A2-662 rev)||--||-- | ||
| + | |- | ||
| + | |3||DT (1C3-661-fwd 1C3 662 rev)||--||-- | ||
| + | |- | ||
| + | |4||spinach-DT(1C3-661-fwd 1C3 662 rev)||--||-- | ||
| + | |- | ||
| + | |5||Pcon-antisense-spinach-DT(1C3-66E fwd A2-661 rev)||--||-- | ||
| + | |- | ||
| + | |6||100bp ladder||--||-- | ||
| + | |} | ||
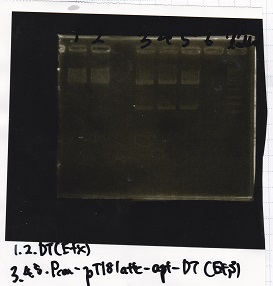
| + | [[File:igku_9251a.jpg]]<br> | ||
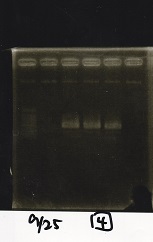
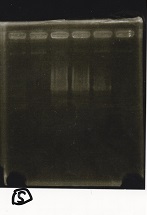
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |2||100bp ladder||--||-- | ||
| + | |- | ||
| + | |3||Ptet (1C3 66E fwd-1C3 660 rev)||--||-- | ||
| + | |- | ||
| + | |4||Pcon-attenuator(1C3-660 fwd A2-661 rev)||--||-- | ||
| + | |- | ||
| + | |5||DT(1C3 662 fwd-1C3 661 rev)||--||-- | ||
| + | |- | ||
| + | |6||Pcon-attenuator-aptamer-DT(1C3 66E fwd-A2-661-rev)||--||-- | ||
| + | |- | ||
| + | |7||100bp ladder||--||-- | ||
| + | |} | ||
| + | [[File:igku_9251b.jpg]]<br> | ||
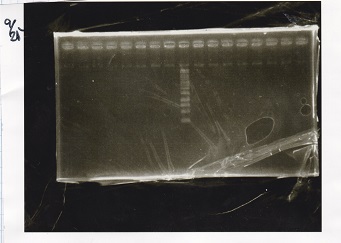
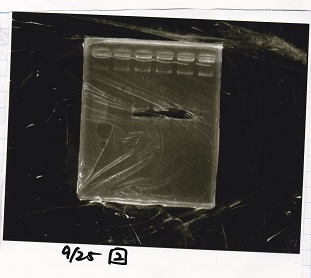
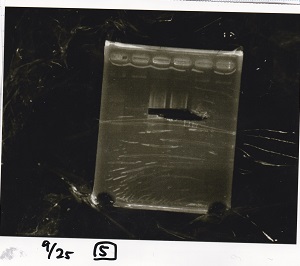
===Colony PCR=== | ===Colony PCR=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| Line 64: | Line 100: | ||
|17||Pcon-PT181 attenuator-pSB1C3-8||--||-- | |17||Pcon-PT181 attenuator-pSB1C3-8||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_9252.jpg]]<br> |
</div> | </div> | ||
| Line 73: | Line 109: | ||
!Name||Sample||Competent Cells||Total||Plate | !Name||Sample||Competent Cells||Total||Plate | ||
|- | |- | ||
| - | |Plac+tet aptamer12+Pcon tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Plac+tet aptamer12+Pcon tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-pT181attenuator-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-pT181attenuator-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-tet aptamer12-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-tet aptamer12-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-Spinach-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-Spinach-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-pT181antisense+DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-pT181antisense+DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-pT181attenuator+tet aptamer12-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-pT181attenuator+tet aptamer12-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon tetR-DT+Ptet+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon tetR-DT+Pcon-GFP-DT+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon tetR-DT+Pcon-GFP-DT+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-tet aptamer12-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-tet aptamer12-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-pT181attenuator-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-pT181attenuator-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-tet aptamer12-DT+Ptet-pT181antisense+Spinach-DT+Pcon-RBS-GFP-DT+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-tet aptamer12-DT+Ptet-pT181antisense+Spinach-DT+Pcon-RBS-GFP-DT+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-tet aptamer12-DT+Pcon-pT181antisense+Spinach-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-tet aptamer12-DT+Pcon-pT181antisense+Spinach-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
| - | |Pcon-tet aptamer12-DT+Ptet-pT181antisense+Spinach-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||- | + | |Pcon-tet aptamer12-DT+Ptet-pT181antisense+Spinach-DT+Pcon-pT181attenuator+RBS-GFP-DT||1µL||10µL||11µL||-- |
|- | |- | ||
|} | |} | ||
| Line 106: | Line 142: | ||
!Name||Sample||Competent Cells||Total||Plate | !Name||Sample||Competent Cells||Total||Plate | ||
|- | |- | ||
| - | |aptamer12-DT(EcoRI+ | + | |aptamer12-DT(EcoRI+XbaI)+Pcon-pT181attenuator(EcoRI+SpeI)||2µL||20µL||22µL||-- |
|- | |- | ||
| - | |Ptet( | + | |Ptet(SpeI+PstI)+RBS-GFP-DT(XbaI+PstI))||2µL||20µL||22µL||-- |
|- | |- | ||
|} | |} | ||
| + | </div> | ||
| + | |||
| + | ===Ligation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||9/25 aptamer12_1R-DT(EcoRI&XbaI)||4.5 µL||Pcon-pT181 attenuator(EcoRI&SpeI)||7.7 µL||6.1 µL | ||
| + | |- | ||
| + | |experiment||9/17 pSB1C3(EcoRI&SpeI)||2.0 µL||9/3 pT181 attenuator(EcoRI&SpeI)||14 µL||8 µL | ||
| + | |- | ||
| + | |experiment||9/25 Pcon-pT181 attenuator(EcoRI&XbaI)||4.5 µL||9/25 RBS-GFP-DT(EcoRI&SpeI)||19.3 µL||11.9 µL | ||
| + | |- | ||
| + | |experiment||9/25 Pcon-RBS-GFP-DT(EcoRI&XbaI)||4.2 µL||Pcon-RBS-tetR-DT(EcoRI&SpeI)||20 µL||12.1 µL | ||
| + | |||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">nakamoto> | ||
| + | {| class="wikitable" | ||
| + | ! ||9/24 Pcon-attenuator-aptamer-DT(µL)||EcoRI(µL)||SpeI(µL)||Buffer(µL)||MilliQ(µL)||total(µL) | ||
| + | |- | ||
| + | |2 cuts||8.6||1||1||3||16.4||30 | ||
| + | |- | ||
| + | |NC||0.4||0||0||1||8.6||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||9/11 DT(µL)||EcoRI(µL)||XbaI(µL)||Buffer(µL)||BSA(µL)||MilliQ(µL)||total(µL) | ||
| + | |- | ||
| + | |2 cuts||10.4||1||1||3||3||11.6||30 | ||
| + | |- | ||
| + | |NC||0.5||0||0||1||1||7.5||10 | ||
| + | |} | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |DT ||-- | ||
| + | |- | ||
| + | |pT181attenuator ||-- | ||
| + | |- | ||
| + | |Pcon-RBS-tetR-DT ||-- | ||
| + | |} | ||
| + | </div> | ||
| + | |||
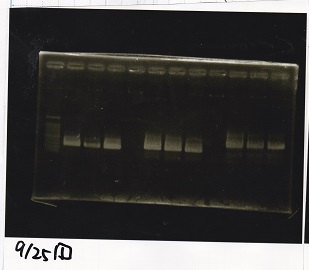
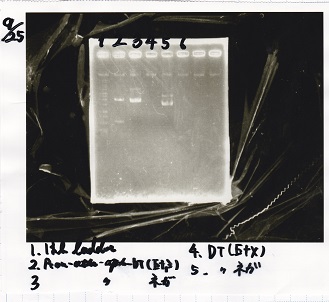
| + | ===Gel Extraction=== | ||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||100bp ladder||-- | ||
| + | |- | ||
| + | |2||rowspan=3|Pcon-pT181antisense||rowspan=3|-- | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4 | ||
| + | |- | ||
| + | |5||--||-- | ||
| + | |- | ||
| + | |6||rowspan=3|DT||rowspan=3|-- | ||
| + | |- | ||
| + | |7 | ||
| + | |- | ||
| + | |8 | ||
| + | |- | ||
| + | |9||--||-- | ||
| + | |- | ||
| + | |10||rowspan=3|Spinach-DT||rowspan=3|-- | ||
| + | |- | ||
| + | |11 | ||
| + | |- | ||
| + | |12 | ||
| + | |} | ||
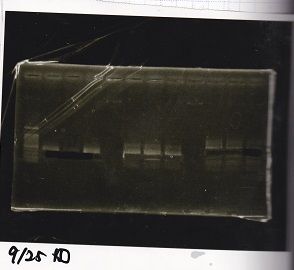
| + | [[File:igku_9253.jpg]]<br> | ||
| + | [[File:igku_9254.jpg]]<br> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | <br> | ||
| + | <div class="experiment"> | ||
| + | <span class="author"></span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||100bp ladder||-- | ||
| + | |- | ||
| + | |3||Pcon-antisense-spinach-DT(E-1A)||-- | ||
| + | |- | ||
| + | |4||Pcon-antisense-spinach-DT(E-1A)||-- | ||
| + | |- | ||
| + | |5||Pcon-antisense-spinach-DT(E-1A)||-- | ||
| + | |- | ||
| + | |} | ||
| + | [[File:igku_9255.jpg]]<br> | ||
| + | [[File:igku_9256.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |7||100bp ladder||-- | ||
| + | |- | ||
| + | |9||Ptet(E-0)||-- | ||
| + | |- | ||
| + | |10||Ptet(E-0)||-- | ||
| + | |- | ||
| + | |11||Ptet(E-0)||-- | ||
| + | |} | ||
| + | [[File:igku_9257.jpg]]<br> | ||
| + | [[File:igku_9258.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |13||100bp ladder||-- | ||
| + | |- | ||
| + | |15||Pcon-attenuator(0-1A)||-- | ||
| + | |- | ||
| + | |16||Pcon-attenuator(0-1A)||-- | ||
| + | |- | ||
| + | |17||Pcon-attenuator(0-1A)||-- | ||
| + | |} | ||
| + | [[File:igku_9259.jpg]]<br>||-- | ||
| + | [[File:igku_92510.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |19||100bp ladder||-- | ||
| + | |- | ||
| + | |21||Pcon-attenuator-aptamer-DT(E-1A)||-- | ||
| + | |- | ||
| + | |22||Pcon-attenuator-aptamer-DT(E-1A)||-- | ||
| + | |- | ||
| + | |23||Pcon-attenuator-aptamer-DT(E-1A)||-- | ||
| + | |- | ||
| + | |} | ||
| + | [[File:igku_92511.jpg]]<br> | ||
| + | [[File:igku_92512.jpg]]<br> | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |DT(2-1)||24.9||1.73||0.37 | ||
| + | |- | ||
| + | |Ptet(E-0)||12.8||1.59||0.90 | ||
| + | |- | ||
| + | |Pcon-antisense(E-2A)||16.5||1.72||1.07 | ||
| + | |- | ||
| + | |Pcon-attenuator-aptamer-DT(E-1A)||22.1||1.75||0.94 | ||
| + | |- | ||
| + | |Pcon-antisense-spinach-DT(E-1A)||31.0||1.78||1.15 | ||
| + | |- | ||
| + | |spinach-DT(1-2)||23.4||1.85||1.11 | ||
| + | |- | ||
| + | |Pcon-attenuator(0-1A)||22.5||1.76||0.46 | ||
| + | |- | ||
| + | |DT(1-2)||25.1||1.78||1.21 | ||
| + | |} | ||
| + | |||
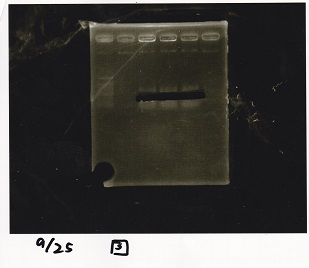
| + | ===Electrophoresis=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author"> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||1kbp ladder||--||-- | ||
| + | |- | ||
| + | |2||Pcon-pT181 attenuator-aptamer 12_1R-DT||EcoRI||SpeI | ||
| + | |- | ||
| + | |3||Pcon-pT181 attenuator-aptamer 12_1R-DT||--||-- | ||
| + | |- | ||
| + | |4||DT||EcoRI||XbaI | ||
| + | |- | ||
| + | |5||DT||--||-- | ||
| + | |- | ||
| + | |} | ||
| + | [[File:igku_92513.jpg]]<br> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||DT||EcoRI+XbaI | ||
| + | |- | ||
| + | |2||DT||EcoRI+XbaI | ||
| + | |- | ||
| + | |3||Pcon-pT181 attenuator-aptamer12_1R-DT||EcoRI+SpeI | ||
| + | |- | ||
| + | |4||Pcon-pT181 attenuator-aptamer12_1R-DT||EcoRI+SpeI | ||
| + | |- | ||
| + | |5||Pcon-pT181 attenuator-aptamer12_1R-DT||EcoRI+SpeI | ||
| + | |} | ||
| + | [[File:igku_92514.jpg]]<br> | ||
| + | [[File:igku_92515.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |Pcon-pT181 attenuator-aptamer12_1R-DT(EcoRI&SpeI)||11.8||1.80||0.46 | ||
| + | |- | ||
| + | |DT(EcoRI+XbaI)||42.6||1.84||0.98 | ||
| + | |} | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |Pcon-pT181anntisense-spinach-DT(4k5)||- | ||
| + | |} | ||
</div> | </div> | ||
| Line 118: | Line 373: | ||
<!-- こっから --> | <!-- こっから --> | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Nakamoto and Tatsui</span> |
{| class="wikitable" | {| class="wikitable" | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |experiment||9/ | + | |experiment||9/24 pSB1C3(EcoRI+Spel)||10µL||9/25 PconpT181attenuator-aptamer12-1R-DT(EcoRI+Spel)||5.9µL||8µL |
| + | |} | ||
| + | </div> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
| + | ===PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano | ||
| + | {| class="wikitable" | ||
| + | !800pg/μL DT||KOD plus||10x buffer||dNTP||MgSO4||1C3_GG2_fwd||iC3_GG1_rev||MilliQ||total | ||
|- | |- | ||
| - | | | + | |1||0.5||2.5||2.5||1.5||0.75||0.75||15.5||25 |
|} | |} | ||
| - | | | + | {| class="wikitable" |
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
|- | |- | ||
| - | | | + | |94°C||98°C||57°C||68°C||-- |
| + | |- | ||
| + | |2min||10s||30s||24s||30cycles | ||
| + | |} | ||
| + | ===Transformation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||Plate | ||
| + | |- | ||
| + | |aptamer12-1R-DT||CP | ||
| + | |- | ||
| + | |pSB1C3(EcoRI+SpeI)+pT181attenuator(EcoRI+Spal)||CP | ||
| + | |- | ||
| + | |Pcon-pT181attenuator(EcoRI+XbaI)+RBS-GFP-DT(EcoRI+SpeI)||Amp | ||
| + | |- | ||
| + | |Pcon-RBS-GFP-DT(EcoRI+XbaI)+Pcon-RBS-tetR-DT(EcoRI+SpeI)||Amp | ||
| + | |- | ||
| + | |pSB1C3(EcoRI+SpeI)+Pcon-pT181attenuator-aptamer12-1R-DT(EcoRI+SpeI)||CP | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||DT(2-1)||--||-- | ||
| + | |} | ||
| + | [[File:igku_92516.jpg]]<br> | ||
</div> | </div> | ||
<!-- ここまでをコピーしてね --> | <!-- ここまでをコピーしてね --> | ||
| + | |||
| + | ===Column Refining=== | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |8/25 DT(2-1)||--||--||-- | ||
| + | |} | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author"></span> | ||
| + | Concentrate PCR product by evaporator until volume become less than 3µL, add all sulution and pipetting. </div> | ||
| + | {| class="wikitable" | ||
| + | !9/25 DT(2-1)||10*cut smart Buffer||BsaI-HF||MilliQ||total | ||
| + | |- | ||
| + | |3.0 µL||2.0 µL||0.3 µL||14.7 µL||20.0 µL | ||
| + | |} | ||
| + | Incubate 37°C 12hour | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |Pcon-GFP-DT||LB(Amp) | ||
| + | |- | ||
| + | |RBS-GFP-DT||LB(CP) | ||
| + | |- | ||
| + | |Pcon-spinach-DT||LB(Amp) | ||
| + | |- | ||
| + | |Pcon-tetRaptamer-DT||LB(Amp) | ||
| + | |- | ||
| + | |Pcon-attenuator-DT||LB(Amp) | ||
| + | |- | ||
| + | |spinach-DT||LB(CP) | ||
| + | |- | ||
| + | |Pcon-antisense-spinach-DT||LB(Amp) | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | ===Plating=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | Mix Liquid Culture 1mL and LB(+Amp), incubate 37°C 30min</div> | ||
| + | OD600-1.445</div> | ||
| + | {| class="wikitable" | ||
| + | !Sample||Use plate||The number of plate | ||
| + | |- | ||
| + | |Pcon-GFP-DT||M9(+Amp)||3 | ||
| + | |- | ||
| + | |Pcon-GFP-DT||LB(+Amp)||3 | ||
| + | |} | ||
| + | </div> | ||
| + | incubate 37°C | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <!-- ここから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||base pair | ||
| + | |- | ||
| + | |9/24 GGA-1(1~8)||3177 | ||
| + | |- | ||
| + | |9/24 GGA-2(1~8)||3189 | ||
| + | |- | ||
| + | |9/24 GGA-3(1~8)||2989 | ||
| + | |- | ||
| + | |9/24 GGA-4(1~8)||2939 | ||
| + | |- | ||
| + | |9/24 GGA-7(1)||2489 | ||
| + | |- | ||
| + | |GGA-16(1)||2717 | ||
| + | |- | ||
| + | |RBS-GFP-DT||-- | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||3min15s||30cycles | ||
| + | |} | ||
| + | </div> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
| + | ===RNA Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span><BR> | ||
| + | sumple 0.25mL<BR> | ||
| + | Add 1mLISOGEN-LS<BR> | ||
| + | Lysis or homogenization<BR> | ||
| + | Store for 5 min, at room temperature<BR> | ||
| + | Add 0.2mL Chloroform<BR> | ||
| + | Shake vigorously for 15sec.<BR> | ||
| + | Store for 2~3min.at room temperature<BR> | ||
| + | Centrifuge 12K*g for 15min. at 4°C<BR> | ||
| + | Extract aqueous phase<BR> | ||
| + | Add 0.5mL isopropanol<BR> | ||
| + | Store for 5~10min. at room temperature<BR> | ||
| + | Centrifuge 12K*g for 10min. at 4°C<BR> | ||
| + | Remove aqueous phase<BR> | ||
| + | Add at least 1mL 70% ethanol<BR> | ||
| + | Vortex<BR> | ||
| + | Centrifuge 7.5K*g for 5min. at 4°C<BR> | ||
| + | Remove aqueous phase<BR> | ||
| + | Dry briefly<BR> | ||
| + | Add ddH2O, TE(pH8.0) or 0.5% SDS<BR> | ||
| + | Dissolve<BR> | ||
| + | Total RNA solution<BR> | ||
| + | {|class="wikitable" | ||
| + | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |Pcon-RBS-GFP-DT||2783||1.95||1.48 | ||
| + | |} | ||
| + | </div> | ||
| + | Add 91.2µL, Store at freezer<BR> | ||
| + | Total 250µL</div> | ||
| + | |||
| + | ===cDNA Synthesis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !volume||7 µL | ||
| + | |- | ||
| + | |7*cDNA Wipcout Buffer||1 µL | ||
| + | |- | ||
| + | |Template RNA(9/25 Pcon-RBS-GFP-DT)||3 µL | ||
| + | |- | ||
| + | |RNase-frec water||4 µL | ||
| + | |} | ||
| + | Incubate 42°C 2min, then on ice immediately<BR> | ||
| + | Add <BR> | ||
| + | {| class="wikitable" | ||
| + | !5*Quantiscript Reverse Transcriptase||0.5 µL | ||
| + | |- | ||
| + | |5*Quantiscript RT Buffer||2 µL | ||
| + | |- | ||
| + | |RT Primer Mix||0.5µL | ||
| + | |} | ||
| + | Incubate 42°C 15min, then incubate 95°C 3min | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||Pcon-RBS-GFP-DT(RNA)||--||-- | ||
| + | |} | ||
| + | [[File:igku_92517.jpg]]<br> | ||
| + | </div> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
| + | ===Ligation=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||9/13 Pcon-pT181-attenuator(SpeI&PstI)||3.5µL||9/25 RBS-GFP-DT(XbaI&PstI)||13.5µL||8.5 µL | ||
| + | |} | ||
| + | </div> | ||
Latest revision as of 19:26, 27 September 2013
Sep 25
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Pcon-antisense (1C3-66E-fwd A2-662 rev) | -- | -- |
| 3 | DT (1C3-661-fwd 1C3 662 rev) | -- | -- |
| 4 | spinach-DT(1C3-661-fwd 1C3 662 rev) | -- | -- |
| 5 | Pcon-antisense-spinach-DT(1C3-66E fwd A2-661 rev) | -- | -- |
| 6 | 100bp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 2 | 100bp ladder | -- | -- |
| 3 | Ptet (1C3 66E fwd-1C3 660 rev) | -- | -- |
| 4 | Pcon-attenuator(1C3-660 fwd A2-661 rev) | -- | -- |
| 5 | DT(1C3 662 fwd-1C3 661 rev) | -- | -- |
| 6 | Pcon-attenuator-aptamer-DT(1C3 66E fwd-A2-661-rev) | -- | -- |
| 7 | 100bp ladder | -- | -- |
Colony PCR
| Sample | base pair |
|---|---|
| 9/23 Pcon-PT181 attenuator-aptamer12-1R-DT-(5~12) | 859 |
| 9/23 Pcon-PT181 attenuator-RBS-GFP-DT-(5~8) | 1527 |
| 9/23 Pcon-PT181 attenuator-pSB1C3-(5~8) | 601 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94 °C | 94 °C | 55 °C | 68 °C | -- |
| 2 min | 30 s | 30 s | 1min5s | 30 cycles |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | Pcon-PT181 attenuator-aptamer12-1R-DT-5 | -- | -- |
| 2 | Pcon-PT181 attenuator-aptamer12-1R-DT-6 | -- | -- |
| 3 | Pcon-PT181 attenuator-aptamer12-1R-DT-7 | -- | -- |
| 4 | Pcon-PT181 attenuator-aptamer12-1R-DT-8 | -- | -- |
| 5 | Pcon-PT181 attenuator-aptamer12-1R-DT-9 | -- | -- |
| 6 | Pcon-PT181 attenuator-aptamer12-1R-DT-10 | -- | -- |
| 7 | Pcon-PT181 attenuator-aptamer12-1R-DT-11 | -- | -- |
| 8 | Pcon-PT181 attenuator-aptamer12-1R-DT-12 | -- | -- |
| 9 | 1kbp ladder | -- | -- |
| 10 | Pcon-PT181 attenuator-RBS-GFP-DT-5 | -- | -- |
| 11 | Pcon-PT181 attenuator-RBS-GFP-DT-6 | -- | -- |
| 12 | Pcon-PT181 attenuator-RBS-GFP-DT-7 | -- | -- |
| 13 | Pcon-PT181 attenuator-RBS-GFP-DT-8 | -- | -- |
| 14 | Pcon-PT181 attenuator-pSB1C3-5 | -- | -- |
| 15 | Pcon-PT181 attenuator-pSB1C3-6 | -- | -- |
| 16 | Pcon-PT181 attenuator-pSB1C3-7 | -- | -- |
| 17 | Pcon-PT181 attenuator-pSB1C3-8 | -- | -- |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| Plac+tet aptamer12+Pcon tetR-DT+Ptet+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-pT181attenuator-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-tet aptamer12-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-Spinach-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-pT181antisense+DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-pT181attenuator+tet aptamer12-DT+Pcon-tetR-DT+Ptet+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon tetR-DT+Ptet+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon tetR-DT+Pcon-GFP-DT+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-tet aptamer12-DT+Pcon-pT181attenuator+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-pT181attenuator-DT+Pcon-pT181attenuator+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-tet aptamer12-DT+Ptet-pT181antisense+Spinach-DT+Pcon-RBS-GFP-DT+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-tet aptamer12-DT+Pcon-pT181antisense+Spinach-DT+Pcon-pT181attenuator+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Pcon-tet aptamer12-DT+Ptet-pT181antisense+Spinach-DT+Pcon-pT181attenuator+RBS-GFP-DT | 1µL | 10µL | 11µL | -- |
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| aptamer12-DT(EcoRI+XbaI)+Pcon-pT181attenuator(EcoRI+SpeI) | 2µL | 20µL | 22µL | -- |
| Ptet(SpeI+PstI)+RBS-GFP-DT(XbaI+PstI)) | 2µL | 20µL | 22µL | -- |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/25 aptamer12_1R-DT(EcoRI&XbaI) | 4.5 µL | Pcon-pT181 attenuator(EcoRI&SpeI) | 7.7 µL | 6.1 µL |
| experiment | 9/17 pSB1C3(EcoRI&SpeI) | 2.0 µL | 9/3 pT181 attenuator(EcoRI&SpeI) | 14 µL | 8 µL |
| experiment | 9/25 Pcon-pT181 attenuator(EcoRI&XbaI) | 4.5 µL | 9/25 RBS-GFP-DT(EcoRI&SpeI) | 19.3 µL | 11.9 µL |
| experiment | 9/25 Pcon-RBS-GFP-DT(EcoRI&XbaI) | 4.2 µL | Pcon-RBS-tetR-DT(EcoRI&SpeI) | 20 µL | 12.1 µL |
Restriction Enzyme Digestion
| 9/24 Pcon-attenuator-aptamer-DT(µL) | EcoRI(µL) | SpeI(µL) | Buffer(µL) | MilliQ(µL) | total(µL) | |
|---|---|---|---|---|---|---|
| 2 cuts | 8.6 | 1 | 1 | 3 | 16.4 | 30 |
| NC | 0.4 | 0 | 0 | 1 | 8.6 | 10 |
| 9/11 DT(µL) | EcoRI(µL) | XbaI(µL) | Buffer(µL) | BSA(µL) | MilliQ(µL) | total(µL) | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 10.4 | 1 | 1 | 3 | 3 | 11.6 | 30 |
| NC | 0.5 | 0 | 0 | 1 | 1 | 7.5 | 10 |
Liquid Culture
| Sample | medium |
|---|---|
| DT | -- |
| pT181attenuator | -- |
| Pcon-RBS-tetR-DT | -- |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | Pcon-pT181antisense | -- |
| 3 | ||
| 4 | ||
| 5 | -- | -- |
| 6 | DT | -- |
| 7 | ||
| 8 | ||
| 9 | -- | -- |
| 10 | Spinach-DT | -- |
| 11 | ||
| 12 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 3 | Pcon-antisense-spinach-DT(E-1A) | -- |
| 4 | Pcon-antisense-spinach-DT(E-1A) | -- |
| 5 | Pcon-antisense-spinach-DT(E-1A) | -- |
| Lane | DNA | Enzyme |
|---|---|---|
| 7 | 100bp ladder | -- |
| 9 | Ptet(E-0) | -- |
| 10 | Ptet(E-0) | -- |
| 11 | Ptet(E-0) | -- |
| Lane | DNA | Enzyme |
|---|---|---|
| 13 | 100bp ladder | -- |
| 15 | Pcon-attenuator(0-1A) | -- |
| 16 | Pcon-attenuator(0-1A) | -- |
| 17 | Pcon-attenuator(0-1A) | -- |
| Lane | DNA | Enzyme |
|---|---|---|
| 19 | 100bp ladder | -- |
| 21 | Pcon-attenuator-aptamer-DT(E-1A) | -- |
| 22 | Pcon-attenuator-aptamer-DT(E-1A) | -- |
| 23 | Pcon-attenuator-aptamer-DT(E-1A) | -- |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| DT(2-1) | 24.9 | 1.73 | 0.37 |
| Ptet(E-0) | 12.8 | 1.59 | 0.90 |
| Pcon-antisense(E-2A) | 16.5 | 1.72 | 1.07 |
| Pcon-attenuator-aptamer-DT(E-1A) | 22.1 | 1.75 | 0.94 |
| Pcon-antisense-spinach-DT(E-1A) | 31.0 | 1.78 | 1.15 |
| spinach-DT(1-2) | 23.4 | 1.85 | 1.11 |
| Pcon-attenuator(0-1A) | 22.5 | 1.76 | 0.46 |
| DT(1-2) | 25.1 | 1.78 | 1.21 |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kbp ladder | -- | -- |
| 2 | Pcon-pT181 attenuator-aptamer 12_1R-DT | EcoRI | SpeI |
| 3 | Pcon-pT181 attenuator-aptamer 12_1R-DT | -- | -- |
| 4 | DT | EcoRI | XbaI |
| 5 | DT | -- | -- |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | DT | EcoRI+XbaI |
| 2 | DT | EcoRI+XbaI |
| 3 | Pcon-pT181 attenuator-aptamer12_1R-DT | EcoRI+SpeI |
| 4 | Pcon-pT181 attenuator-aptamer12_1R-DT | EcoRI+SpeI |
| 5 | Pcon-pT181 attenuator-aptamer12_1R-DT | EcoRI+SpeI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-pT181 attenuator-aptamer12_1R-DT(EcoRI&SpeI) | 11.8 | 1.80 | 0.46 |
| DT(EcoRI+XbaI) | 42.6 | 1.84 | 0.98 |
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-pT181anntisense-spinach-DT(4k5) | - |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/24 pSB1C3(EcoRI+Spel) | 10µL | 9/25 PconpT181attenuator-aptamer12-1R-DT(EcoRI+Spel) | 5.9µL | 8µL |
PCR
| 800pg/μL DT | KOD plus | 10x buffer | dNTP | MgSO4 | 1C3_GG2_fwd | iC3_GG1_rev | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 24s | 30cycles |
Transformation
| Name | Plate |
|---|---|
| aptamer12-1R-DT | CP |
| pSB1C3(EcoRI+SpeI)+pT181attenuator(EcoRI+Spal) | CP |
| Pcon-pT181attenuator(EcoRI+XbaI)+RBS-GFP-DT(EcoRI+SpeI) | Amp |
| Pcon-RBS-GFP-DT(EcoRI+XbaI)+Pcon-RBS-tetR-DT(EcoRI+SpeI) | Amp |
| pSB1C3(EcoRI+SpeI)+Pcon-pT181attenuator-aptamer12-1R-DT(EcoRI+SpeI) | CP |
Electrophoresis
Column Refining
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/25 DT(2-1) | -- | -- | -- |
Restriction Enzyme Digestion
Concentrate PCR product by evaporator until volume become less than 3µL, add all sulution and pipetting.
| 9/25 DT(2-1) | 10*cut smart Buffer | BsaI-HF | MilliQ | total |
|---|---|---|---|---|
| 3.0 µL | 2.0 µL | 0.3 µL | 14.7 µL | 20.0 µL |
Incubate 37°C 12hour
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-GFP-DT | LB(Amp) |
| RBS-GFP-DT | LB(CP) |
| Pcon-spinach-DT | LB(Amp) |
| Pcon-tetRaptamer-DT | LB(Amp) |
| Pcon-attenuator-DT | LB(Amp) |
| spinach-DT | LB(CP) |
| Pcon-antisense-spinach-DT | LB(Amp) |
Plating
Mix Liquid Culture 1mL and LB(+Amp), incubate 37°C 30min
| Sample | Use plate | The number of plate |
|---|---|---|
| Pcon-GFP-DT | M9(+Amp) | 3 |
| Pcon-GFP-DT | LB(+Amp) | 3 |
</div> incubate 37°C
Colony PCR
| Sample | base pair |
|---|---|
| 9/24 GGA-1(1~8) | 3177 |
| 9/24 GGA-2(1~8) | 3189 |
| 9/24 GGA-3(1~8) | 2989 |
| 9/24 GGA-4(1~8) | 2939 |
| 9/24 GGA-7(1) | 2489 |
| GGA-16(1) | 2717 |
| RBS-GFP-DT | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 3min15s | 30cycles |
RNA Extraction
sumple 0.25mL
Add 1mLISOGEN-LS
Lysis or homogenization
Store for 5 min, at room temperature
Add 0.2mL Chloroform
Shake vigorously for 15sec.
Store for 2~3min.at room temperature
Centrifuge 12K*g for 15min. at 4°C
Extract aqueous phase
Add 0.5mL isopropanol
Store for 5~10min. at room temperature
Centrifuge 12K*g for 10min. at 4°C
Remove aqueous phase
Add at least 1mL 70% ethanol
Vortex
Centrifuge 7.5K*g for 5min. at 4°C
Remove aqueous phase
Dry briefly
Add ddH2O, TE(pH8.0) or 0.5% SDS
Dissolve
Total RNA solution
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-RBS-GFP-DT | 2783 | 1.95 | 1.48 |
Add 91.2µL, Store at freezer
Total 250µL</div>
cDNA Synthesis
| volume | 7 µL |
|---|---|
| 7*cDNA Wipcout Buffer | 1 µL |
| Template RNA(9/25 Pcon-RBS-GFP-DT) | 3 µL |
| RNase-frec water | 4 µL |
Incubate 42°C 2min, then on ice immediately
Add
| 5*Quantiscript Reverse Transcriptase | 0.5 µL |
|---|---|
| 5*Quantiscript RT Buffer | 2 µL |
| RT Primer Mix | 0.5µL |
Incubate 42°C 15min, then incubate 95°C 3min
Electrophoresis
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/13 Pcon-pT181-attenuator(SpeI&PstI) | 3.5µL | 9/25 RBS-GFP-DT(XbaI&PstI) | 13.5µL | 8.5 µL |
 "
"