Team:SDU-Denmark/Tour51
From 2013.igem.org
Shengry0716 (Talk | contribs) |
|||
| (60 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | |||
{{:Team:SDU-Denmark/core/header| }} | {{:Team:SDU-Denmark/core/header| }} | ||
<html> | <html> | ||
| Line 14: | Line 15: | ||
<p> | <p> | ||
| - | <a class="popupImg alignRight" style="width:130px" href="https://static.igem.org/mediawiki/2013/a/a8/SDU2013_Cloning_1.png" title="Figure 1 - The picture shows the colony PCR result for the cloning and subsequent transformation. The gel picture shows the expected length around 2300 bp including verification primers."> | + | |
| + | <a class="popupImg alignRight" style="width:130px" href="https://static.igem.org/mediawiki/2013/a/a8/SDU2013_Cloning_1.png" title="Figure 1 - The picture shows the colony PCR result for the cloning and subsequent transformation of pSB1C3-Plac-<i>dxs(E.coli)</i>. The gel picture shows the expected length around 2300 bp including verification primers."> | ||
<img src="https://static.igem.org/mediawiki/2013/a/a8/SDU2013_Cloning_1.png" | <img src="https://static.igem.org/mediawiki/2013/a/a8/SDU2013_Cloning_1.png" | ||
| Line 21: | Line 23: | ||
</a> | </a> | ||
| - | <h4>pSB1C3-Plac- | + | <h4>pSB1C3-Plac-<span class="specialWord">dxs (E. coli)</span></h4> |
| + | |||
| + | |||
<span class="intro">The | <span class="intro">The | ||
<span class="specialWord">dxs </span>from | <span class="specialWord">dxs </span>from | ||
| - | <span class="specialWord"> E. coli</span> was already present</span> in parts registry | + | <span class="specialWord"> <span class="specialWord">E. coli</span></span> was already present</span> in parts registry |
(<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K118000">BBa_K118000</a>) | (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K118000">BBa_K118000</a>) | ||
| Line 42: | Line 46: | ||
| - | <span class="intro">The literature suggested</span> ( | + | <span class="sourceReference"><span class="intro">The literature suggested </span></span> |
| + | |||
| + | |||
| + | <span class="tooltip"> | ||
| + | <span class="tooltipHeader">Source:</span> | ||
| + | Y. Zhao et al. Biosynthesis of isoprene in Escherichia Coli via methylerythritol phosphate (MEP) pathway. | ||
| + | </span> | ||
| + | |||
| + | that <span class="specialWord">dxs</span> from <span class="specialWord">B. subtilis</span> performed better than its <span class="specialWord">E. coli</span> counterpart. Therefore, a colony PCR was performed on the <span class="specialWord">B. subtilis</span> strain 168. USER cloning was used to produce the construct pSB1C3-Plac-dxs <span class="specialWord">(B. subtilis)</span>. Our tests of the construct unfortunately showed four restriction sites within the coding sequence <b>(Fig. 2)</b>. | ||
</p> | </p> | ||
<p> | <p> | ||
| - | <span class="intro">Site directed mutagenesis</span> was performed to remove the restriction sites, which ensured that the gene complied with iGEM standards. The gel picture shows the expected length around 2000 bp <b>(Fig. 3)</b>. Full confirmed sequence can be found by following this link ( | + | <span class="intro">Site directed mutagenesis</span> was performed to remove the restriction sites, which ensured that the gene complied with iGEM standards. The gel picture shows the expected length around 2000 bp <b>(Fig. 3)</b>. Full confirmed sequence can be found by following this link (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088011" title="">BBa_K1088011</a>). |
</p> | </p> | ||
| + | |||
<br> | <br> | ||
<p> | <p> | ||
| - | <h4>pSB1C3- | + | <h4>pSB1C3-lacI-Plac-<span class="specialWord">dxs (B. subtilis)</span></h4> |
| - | <span class="intro">Then, our focus moved to expression control:</span> Subsequent testing proved that the inherent amount of LacI (the repressor of the lactose promoter) in MG1655 was insufficient in the presence of our construct in a high-copy plasmid (see characterization). Therefore, | + | <span class="intro">Then, our focus moved to expression control:</span> Subsequent testing proved that the inherent amount of LacI (the repressor of the lactose promoter) in MG1655 was insufficient in the presence of our construct in a high-copy plasmid (see characterization). Therefore, <span class="specialWord">lacI</span> with an LVA tag was taken from parts registry (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_C0012">BBa_C0012</a>) and cloned into our construct with its own promoter and terminator. pSB1C3-Pcon-<span class="specialWord">lacI:LVA</span>-term-Plac-<span class="specialWord">dxs (B. subtilis)</span> was born. The gel-picture shows the result for the test digestion. Linearized plasmid has the expected length around 5500 bp, and the EcoRI and PstI cut plasmid has expected lengths around 2000 bp and 3500 bp <b>(Fig. 4)</b>. This, too, was sequenced and may be found here (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088013" title="">BBa_K1088013</a>). The brick allows us to overcome the first rate-limiting step of the MEP pathway in a regulatable manner. |
</p> | </p> | ||
| - | + | <div class="imageGallery galleryMedium alignCenter" style="height: 140px;"> | |
| - | + | ||
| - | <div class="galleryMedium alignCenter"> | + | |
<a class="galleryImg" target="_blank" | <a class="galleryImg" target="_blank" | ||
| - | href="https://static.igem.org/mediawiki/2013/f/f6/SDU2013_Cloning_2.png" title="Figure 2 - USER cloning was used to produce the construct pSB1C3-Plac-dxs (B. subtilis). Our tests of the construct showed unfortunately two restriction sites within the coding sequence."> | + | href="https://static.igem.org/mediawiki/2013/f/f6/SDU2013_Cloning_2.png" title="Figure 2 - USER cloning was used to produce the construct pSB1C3-Plac-<i>dxs (B. subtilis)</i>. Our tests of the construct showed unfortunately two restriction sites within the coding sequence."> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/2/24/SDU2013_Small_Cloning_2.png"> |
| - | + | Figure 2. | |
</a> | </a> | ||
<a class="galleryImg" target="_blank" | <a class="galleryImg" target="_blank" | ||
| - | href="https://static.igem.org/mediawiki/2013/c/ce/SDU2013_Cloning_3.png" title="Figure 3 - Site directed mutagenesis was performed to remove the restriction sites, which ensured that the gene complied with iGEM standards. The gel picture shows the expected length around 2000 bp."> | + | href="https://static.igem.org/mediawiki/2013/c/ce/SDU2013_Cloning_3.png" title="Figure 3 - Site directed mutagenesis was performed to remove the restriction sites in <i>dxs(B. subtilis)</i>, which ensured that the gene complied with iGEM standards. The gel picture shows the expected length around 2000 bp."> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/6/66/SDU2013_Small_Cloning_3.png"> |
| - | + | Figure 3. | |
</a> | </a> | ||
<a class="galleryImg" target="_blank" | <a class="galleryImg" target="_blank" | ||
| - | href="https://static.igem.org/mediawiki/2013/b/bc/SDU2013_Cloning_4.png" title="Figure 4 - The gel-picture shows the result for the test digestion. Linearized plasmid has the expected length around 5500 bp, and the EcoRI and PstI cut plasmid has expected lengths around 2000 bp and 3500 bp."> | + | href="https://static.igem.org/mediawiki/2013/b/bc/SDU2013_Cloning_4.png" title="Figure 4 - The gel-picture shows the result for the test digestion of pSB1C3-<i>LacI:LVA</i>-Plac-<i>dxs(B. subtilis)</i>. Linearized plasmid has the expected length around 5500 bp, and the EcoRI and PstI cut plasmid has expected lengths around 2000 bp and 3500 bp."> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/5/56/SDU2013_Small_Cloning_4.png"> |
| - | + | Figure 4. | |
</a> | </a> | ||
| - | + | | |
| - | + | ||
| - | + | ||
</div> | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br><br> | <br><br> | ||
| - | |||
| - | |||
| Line 102: | Line 103: | ||
<p> | <p> | ||
| - | + | <a class="popupImg alignRight" style="width:200px" href="https://static.igem.org/mediawiki/2013/5/52/SDU2013_Cloning_5.png" title="Figure 5 - The gel picture shows colony PCR result for the transformation with pSB1C3-Pcon-<i>LacI(N)<i/>-term-Plac-<i>dxs(B.subtilis)</i>. A band appeared just above 3000 bp as expected for this construct."> | |
| - | <a class="popupImg alignRight" style="width:200px" href="https://static.igem.org/mediawiki/2013/5/52/SDU2013_Cloning_5.png" title="Figure 5 - | + | |
<img src="https://static.igem.org/mediawiki/2013/5/52/SDU2013_Cloning_5.png" | <img src="https://static.igem.org/mediawiki/2013/5/52/SDU2013_Cloning_5.png" | ||
| Line 111: | Line 111: | ||
| - | <span class="intro">Additionally, we observed indications</span> that LacI:LVA was an inferior repressor to MG1655’s own LacI. As a consequence, we performed yet another colony PCR to amplify natural LacI, and clone this into our construct in place of LacI:LVA. | + | <span class="intro">Additionally, we observed indications</span> that LacI:LVA was an inferior repressor to MG1655’s own LacI. As a consequence, we performed yet another colony PCR to amplify natural LacI, and clone this into our construct in place of LacI:LVA. The gel picture shows colony PCR result for the transformation with pSB1C3-Pcon-<span class="specialWord">lacI(N)</span>-term-Plac-<span class="specialWord">dxs (B. sub)</span>. A band appeared just above 3000 bp as expected for this construct <b>(Fig. 5)</b>. The confirmed sequence can be found here (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088027" title="">BBa_K1088027</a>). |
| Line 141: | Line 141: | ||
</a> | </a> | ||
| - | <span class="intro">GenStript performed the synthesis</span> of HRT2 polyprenyltranferase with a flagtag added in the end of the sequence, an RBS and an arabinose promoter in front. Unfortunately, the stop codon of the gene was not removed, leaving the tag unfunctionable. The coding sequence of HRT2 was amplified with primers adding prefix and suffix to the sequence. The gel picture shows two bands. The band just above 2000 bp corresponds to the expected length of shipped pUC57 plasmid and the second band around 900 bp is the expected length for the coding HTR2 sequence <b>(Fig. 6)</b>. The basic part was sequenced, and the confirmed sequenced can be found here ( | + | <span class="intro">GenStript performed the synthesis</span> of <span class="specialWord">HRT2</span> polyprenyltranferase with a flagtag added in the end of the sequence, an RBS and an arabinose promoter in front. Unfortunately, the stop codon of the gene was not removed, leaving the tag unfunctionable. The coding sequence of HRT2 was amplified with primers adding prefix and suffix to the sequence. The gel picture shows two bands. The band just above 2000 bp corresponds to the expected length of shipped pUC57 plasmid and the second band around 900 bp is the expected length for the coding HTR2 sequence <b>(Fig. 6)</b>. The basic part was sequenced, and the confirmed sequenced can be found here (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088003" title="">BBa_K1088003</a>). |
| Line 151: | Line 151: | ||
<p> | <p> | ||
| - | <a class="popupImg alignLeft" style="width:130px" href="https://static.igem.org/mediawiki/2013/9/9b/SDU2013_Cloning_7.png" title="Figure 7 - The colony PCR for the transformation with | + | <a class="popupImg alignLeft" style="width:130px" href="https://static.igem.org/mediawiki/2013/9/9b/SDU2013_Cloning_7.png" title="Figure 7 - The colony PCR for the transformation with pSB1C3-Pcon-araC-term-Para-HRT2-FT shows a band just above 2500 bp as expected."> |
<img src="https://static.igem.org/mediawiki/2013/9/9b/SDU2013_Cloning_7.png" style="width:130px" /> | <img src="https://static.igem.org/mediawiki/2013/9/9b/SDU2013_Cloning_7.png" style="width:130px" /> | ||
Figure 7. | Figure 7. | ||
| Line 157: | Line 157: | ||
| - | <span class="intro">In order to assay optimized control</span> of the expression from the arabinose promoter and thus HTR2, we build and added an AraC device ( | + | <span class="intro">In order to assay optimized control</span> of the expression from the arabinose promoter and thus HTR2, we build and added an AraC device (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088017" title="">BBa_K1088017</a>). A colony PCR was performed on MG1655 to obtain <span class="specialWord">araC</span>, which was cloned into the construct with its own promoter and terminator. The construct design was: pSB1C3-Pcon-<span class="specialWord">araC</span>-term-Para-<span class="specialWord">HRT2</span>-FT. The colony PCR for the transformation with the construct shows a band just above 2500 bp as expected <b>(Fig. 7)</b>. The confirmed sequence can be found here (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088016" title="">BBa_K1088016</a>). |
| Line 165: | Line 165: | ||
<br> | <br> | ||
| - | <a class="popupImg alignRight" style="width:160px" href="https://static.igem.org/mediawiki/2013/5/53/SDU2013_Cloning_7.5.png" title="Figure 8"> | + | <a class="popupImg alignRight" style="width:160px" href="https://static.igem.org/mediawiki/2013/5/53/SDU2013_Cloning_7.5.png" title="Figure 8 - The gel picture shows the result of a colony PCR for the cloning of Pcon-araC-term-Para-HRT2-FT into pSB1K3 and subsequent transformation. A band with the expected length around 2500 bp are found"> |
<img src="https://static.igem.org/mediawiki/2013/5/53/SDU2013_Cloning_7.5.png" style="width:160px"> | <img src="https://static.igem.org/mediawiki/2013/5/53/SDU2013_Cloning_7.5.png" style="width:160px"> | ||
Figure 8. | Figure 8. | ||
| Line 191: | Line 191: | ||
| - | <span class="intro">To check the expression profile</span> of our constructs, we used two distinct methods: <b>1)</b> For the dxs construct, protein fusion with a reporter gene, coupled with microscopy or FACS was used, and <b>2)</b> for the HRT2 construct, Northern blots were performed. To see the results of these tests, see Characterization. | + | <span class="intro">To check the expression profile</span> of our constructs, we used two distinct methods: <b>1)</b> For the <span class="specialWord">dxs</span> construct, protein fusion with a reporter gene, coupled with microscopy or FACS was used, and <b>2)</b> for the <span class="specialWord">HRT2</span> construct, Northern blots were performed. To see the results of these tests, see Characterization. |
</p> | </p> | ||
| Line 197: | Line 197: | ||
<p> | <p> | ||
| - | <span class="intro">For our reporter system</span>, we produced pSB1C3-Pcon-dxs <span class="specialWord">(B. subtilis)</span>-AmilCP ( | + | <span class="intro">For our reporter system</span>, we produced pSB1C3-Pcon-<span class="specialWord">dxs</span> <span class="specialWord">(B. subtilis)</span>-AmilCP (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088006" title="">BBa_K1088006</a>). The AmilCP, however, proved to be a very poor fusion reporter gene, and we abandoned this construct to focus our attention on GFP. A number of constructs were successfully made, corresponding to the evolution of our design outlined above <b>(Fig. 9-11)</b>. |
</p> | </p> | ||
| Line 204: | Line 204: | ||
<p> | <p> | ||
| - | <li><span class="intro">pSB1C3- | + | <li><span class="intro">pSB1C3-Plac-<span class="specialWord">dxs</span> <span class="specialWord">(B. subtilis)</span>-linker-GFP</span> was succesfully cloned and sequenced (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088008" title="">BBa_K1088008</a>), as seen on <b>Figure 9.</b> |
</li> | </li> | ||
</p><p> | </p><p> | ||
| - | <li><span class="intro">pSB1C3- | + | <li><span class="intro">pSB1C3-Plac-<span class="specialWord">dxs</span> <span class="specialWord">(E. coli)</span>-linker-GFP</span> was successfully cloned and sequenced (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088007" title="">BBa_K1088007</a>), as seen on <b>Figure 9</b>. The gel picture shows the result of test digest with a band appearing around 5000 bp. The two constructs have been run next to each other. <br></li> |
</p><p> | </p><p> | ||
| - | <li><span class="intro">pSB1C3-LacI:LVA- | + | <li><span class="intro">pSB1C3-<span class="specialWord">LacI:LVA</span>-Plac-<span class="specialWord">dxs</span> <span class="specialWord">(B. Subtilis)</span>-linker-GFP</span> was successfully cloned and sequenced (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088009" title="">BBa_K1088009</a>). <b>Figure 10</b> shows a test digest with the length of the insert corresponding to the expected length around 5000 bp.<br></li> |
</p><p> | </p><p> | ||
| - | <li><span class="intro">pSB1C3- | + | <li><span class="intro">pSB1C3-<span class="specialWord">lacI(N)</span>-Plac-<span class="specialWord">dxs</span> <span class="specialWord">(B. Subtilis)</span>-linker-GFP</span> was successfully cloned and sequenced (<a class="dialogLink" href="http://parts.igem.org/Part:BBa_K1088026" title="">BBa_K1088026</a>). <b>Figure 11</b> shows a test digest with the length of the insert corresponding to the expected length around 4000 bp.<br></li> |
</p> | </p> | ||
| Line 218: | Line 218: | ||
<p> | <p> | ||
| - | <div class=" | + | <div class="imageGallery galleryMedium alignCenter"> |
| - | <a class="galleryImg" target="_blank | + | <a class="galleryImg" target="_blank" href="https://static.igem.org/mediawiki/2013/8/85/SDU2013_Cloning_8.png" title="Figure 9 - The gel picture shows the result for the test digestion of pSB1C3-Plac-<i>dxs(E.coli)</i>-Linker-GFP (first lane) and pSB1C3-Plac-<i>dxs(B.subtilis)</i>-Linker-GFP (second lane). A band of the expected length, 5000 bp, appears in both lanes."> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/7/77/SDU2013_Small_Cloning_8.png"></img>Figure 9.</a> |
| - | Figure 9. | + | |
| - | </a> | + | |
| - | <a class="galleryImg" target="_blank" href="https://static.igem.org/mediawiki/2013/d/de/SDU2013_Cloning_9.png" title="Figure 10"> | + | <a class="galleryImg" target="_blank" href="https://static.igem.org/mediawiki/2013/d/de/SDU2013_Cloning_9.png" title="Figure 10 - The gel picture shows the result for the test digestion of pSB1C3-<i>LacI:LVA</i>-Plac-<i>dxs(B.subtilis)</i>-Linker-GFP. Linearized plasmid has the expected length around 7000 bp and the insert around 5000 bp."> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/1/1e/SDU2013_Small_Cloning_9.png"></img>Figure 10.</a> |
| - | Figure 10. | + | |
| - | </a> | + | |
| - | <a class="galleryImg" target="_blank" href="https://static.igem.org/mediawiki/2013/9/95/SDU2013_Cloning_10.png" title="Figure 11"> | + | <a class="galleryImg" target="_blank" href="https://static.igem.org/mediawiki/2013/9/95/SDU2013_Cloning_10.png" title="Figure 11 - The gel picture shows the result for the test digestion of pSB1C3-<i>LacI(N)</i>-Plac-<i>dxs(B.subtilis)</i>-Linker-GFP. Linearized plasmid has the expected length around 6000 bp and the insert around 4000 bp. "> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/8/88/SDU2013_Small_Cloning_10.png"></img>Figure 11.</a> |
| - | Figure 11. | + | |
| - | </a | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| + | | ||
</div> | </div> | ||
</p> | </p> | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<p> | <p> | ||
Latest revision as of 13:18, 8 January 2014
Cloning
The beauty of creation
To clarify the progression of our cloning, the results will not be presented chronologically, but rather in a logical manner, starting with the simplest constructs. This is not an exhaustive list, but merely a presentation to clarify our work and give insight into our thoughts. Please consult our submitted parts-page (dig deeper), as well as our comprehensive protocols to gain full insight into our work. We have done our utmost to edit the gel photos in a responsible manner. Should questions arise, originals can be found in our protocols.
pSB1C3-Plac-dxs (E. coli)
The dxs from E. coli was already present in parts registry (BBa_K118000) , which simplified our aim to increase expression of the gene. However, the middle part of the brick was unsequenced, so this was an obvious focal point. We successfully completed the sequencing and the full sequence has been entered into part registry (BBa_K1088012). USER cloning was used to clone PLac together with E.coli dxs in order to control the expression of Dxs. The picture shows the colony PCR result for the cloning and subsequent transformation. The gel picture shows the expected length around 2300 bp including verification primers (Fig. 1).
pSB1C3-Plac-dxs (B. subtilis)
The literature suggested Source: Y. Zhao et al. Biosynthesis of isoprene in Escherichia Coli via methylerythritol phosphate (MEP) pathway. that dxs from B. subtilis performed better than its E. coli counterpart. Therefore, a colony PCR was performed on the B. subtilis strain 168. USER cloning was used to produce the construct pSB1C3-Plac-dxs (B. subtilis). Our tests of the construct unfortunately showed four restriction sites within the coding sequence (Fig. 2).Site directed mutagenesis was performed to remove the restriction sites, which ensured that the gene complied with iGEM standards. The gel picture shows the expected length around 2000 bp (Fig. 3). Full confirmed sequence can be found by following this link (BBa_K1088011).
pSB1C3-lacI-Plac-dxs (B. subtilis)
Then, our focus moved to expression control: Subsequent testing proved that the inherent amount of LacI (the repressor of the lactose promoter) in MG1655 was insufficient in the presence of our construct in a high-copy plasmid (see characterization). Therefore, lacI with an LVA tag was taken from parts registry (BBa_C0012) and cloned into our construct with its own promoter and terminator. pSB1C3-Pcon-lacI:LVA-term-Plac-dxs (B. subtilis) was born. The gel-picture shows the result for the test digestion. Linearized plasmid has the expected length around 5500 bp, and the EcoRI and PstI cut plasmid has expected lengths around 2000 bp and 3500 bp (Fig. 4). This, too, was sequenced and may be found here (BBa_K1088013). The brick allows us to overcome the first rate-limiting step of the MEP pathway in a regulatable manner.
 Figure 5.
Additionally, we observed indications that LacI:LVA was an inferior repressor to MG1655’s own LacI. As a consequence, we performed yet another colony PCR to amplify natural LacI, and clone this into our construct in place of LacI:LVA. The gel picture shows colony PCR result for the transformation with pSB1C3-Pcon-lacI(N)-term-Plac-dxs (B. sub). A band appeared just above 3000 bp as expected for this construct (Fig. 5). The confirmed sequence can be found here (BBa_K1088027).
Figure 5.
Additionally, we observed indications that LacI:LVA was an inferior repressor to MG1655’s own LacI. As a consequence, we performed yet another colony PCR to amplify natural LacI, and clone this into our construct in place of LacI:LVA. The gel picture shows colony PCR result for the transformation with pSB1C3-Pcon-lacI(N)-term-Plac-dxs (B. sub). A band appeared just above 3000 bp as expected for this construct (Fig. 5). The confirmed sequence can be found here (BBa_K1088027).
As a final test of this hypothesis, the plasmid was transformed into KG22, an E. coli strain with natural abundance of LacI. This is further discussed in our characterization of LacI on the next page in the tour.
pSB1C3-Para-HRT2
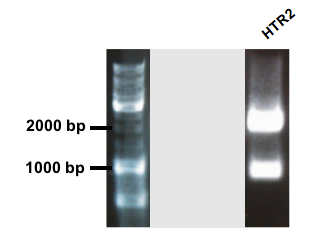 Figure 6.
GenStript performed the synthesis of HRT2 polyprenyltranferase with a flagtag added in the end of the sequence, an RBS and an arabinose promoter in front. Unfortunately, the stop codon of the gene was not removed, leaving the tag unfunctionable. The coding sequence of HRT2 was amplified with primers adding prefix and suffix to the sequence. The gel picture shows two bands. The band just above 2000 bp corresponds to the expected length of shipped pUC57 plasmid and the second band around 900 bp is the expected length for the coding HTR2 sequence (Fig. 6). The basic part was sequenced, and the confirmed sequenced can be found here (BBa_K1088003).
Figure 6.
GenStript performed the synthesis of HRT2 polyprenyltranferase with a flagtag added in the end of the sequence, an RBS and an arabinose promoter in front. Unfortunately, the stop codon of the gene was not removed, leaving the tag unfunctionable. The coding sequence of HRT2 was amplified with primers adding prefix and suffix to the sequence. The gel picture shows two bands. The band just above 2000 bp corresponds to the expected length of shipped pUC57 plasmid and the second band around 900 bp is the expected length for the coding HTR2 sequence (Fig. 6). The basic part was sequenced, and the confirmed sequenced can be found here (BBa_K1088003).
 Figure 7.
In order to assay optimized control of the expression from the arabinose promoter and thus HTR2, we build and added an AraC device (BBa_K1088017). A colony PCR was performed on MG1655 to obtain araC, which was cloned into the construct with its own promoter and terminator. The construct design was: pSB1C3-Pcon-araC-term-Para-HRT2-FT. The colony PCR for the transformation with the construct shows a band just above 2500 bp as expected (Fig. 7). The confirmed sequence can be found here (BBa_K1088016).
Figure 7.
In order to assay optimized control of the expression from the arabinose promoter and thus HTR2, we build and added an AraC device (BBa_K1088017). A colony PCR was performed on MG1655 to obtain araC, which was cloned into the construct with its own promoter and terminator. The construct design was: pSB1C3-Pcon-araC-term-Para-HRT2-FT. The colony PCR for the transformation with the construct shows a band just above 2500 bp as expected (Fig. 7). The confirmed sequence can be found here (BBa_K1088016).
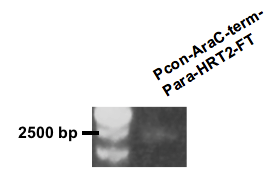 Figure 8.
Figure 8.
We had great difficulty cloning the construct into pSB1A3, and therefore chose pSB1K3 as our plasmid for the production system. The cloning was immediately successful. The gel picture shows the result of a colony PCR with the expected length found at approximately 2500 bp (Fig. 8).
Reporter systems
To check the expression profile of our constructs, we used two distinct methods: 1) For the dxs construct, protein fusion with a reporter gene, coupled with microscopy or FACS was used, and 2) for the HRT2 construct, Northern blots were performed. To see the results of these tests, see Characterization.For our reporter system, we produced pSB1C3-Pcon-dxs (B. subtilis)-AmilCP (BBa_K1088006). The AmilCP, however, proved to be a very poor fusion reporter gene, and we abandoned this construct to focus our attention on GFP. A number of constructs were successfully made, corresponding to the evolution of our design outlined above (Fig. 9-11).
- pSB1C3-Plac-dxs (B. subtilis)-linker-GFP was succesfully cloned and sequenced (BBa_K1088008), as seen on Figure 9.
- pSB1C3-Plac-dxs (E. coli)-linker-GFP was successfully cloned and sequenced (BBa_K1088007), as seen on Figure 9. The gel picture shows the result of test digest with a band appearing around 5000 bp. The two constructs have been run next to each other.
- pSB1C3-LacI:LVA-Plac-dxs (B. Subtilis)-linker-GFP was successfully cloned and sequenced (BBa_K1088009). Figure 10 shows a test digest with the length of the insert corresponding to the expected length around 5000 bp.
- pSB1C3-lacI(N)-Plac-dxs (B. Subtilis)-linker-GFP was successfully cloned and sequenced (BBa_K1088026). Figure 11 shows a test digest with the length of the insert corresponding to the expected length around 4000 bp.
 "
"
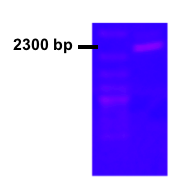 Figure 1.
Figure 1.
 Figure 2.
Figure 2.
 Figure 3.
Figure 3.
 Figure 4.
Figure 4.
 Figure 9.
Figure 9. Figure 10.
Figure 10. Figure 11.
Figure 11.