|
|
| (68 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | {{Template:EPFL2013Header}} | | {{Template:EPFL2013Header}} |
| | | | |
| - | ==Sensing== | + | =='''Sensing'''== |
| | | | |
| | ==Overview== | | ==Overview== |
| | | | |
| - | The idea of this module was to transform bacteria with a plasmid that would contain a promoter which senses a specific signal. Once this promoter senses the signal, it would initiate transcription of an enzyme which degrades the nanoparticle, thus releasing its contents. | + | The idea of this module was to transform bacteria with a plasmid that contains a promoter which senses a specific signal. Once this promoter senses the signal, it would initiate transcription of an enzyme which degrades the nanoparticle, thus releasing its contents. |
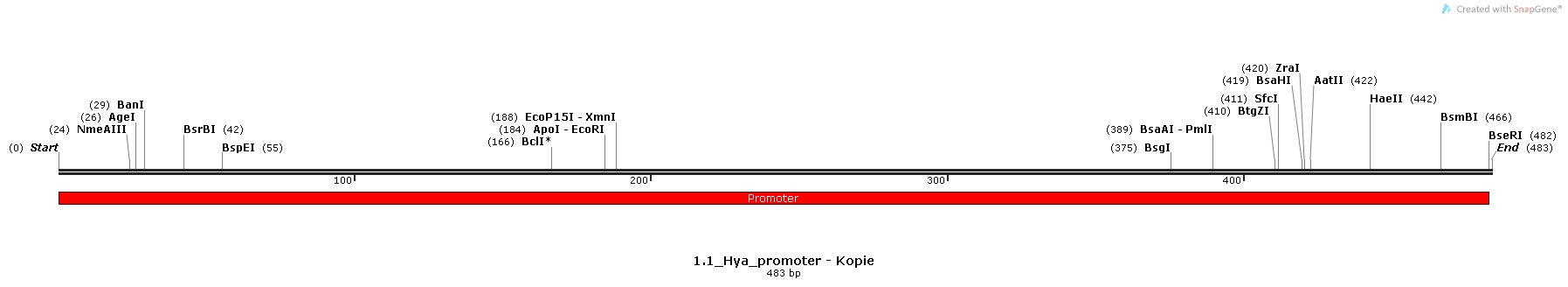
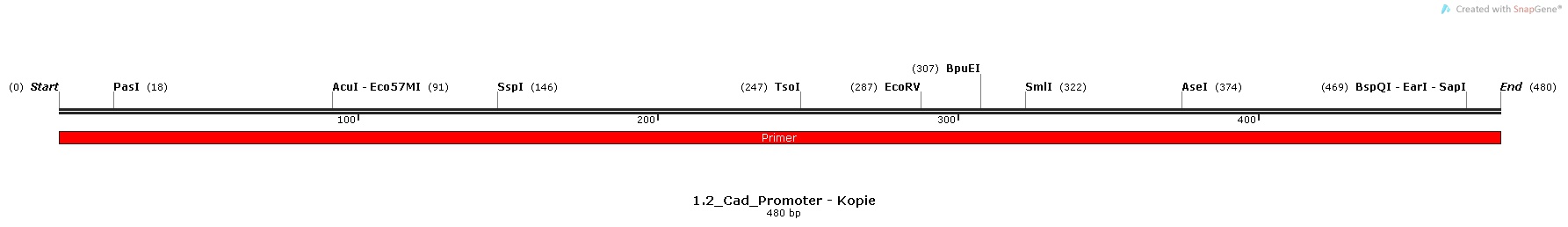
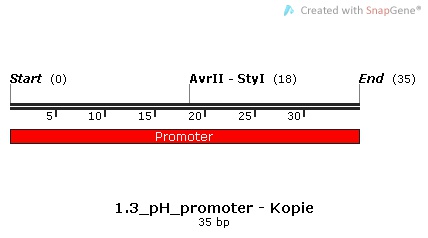
| | We decided to use pH as the specific trigger that activates the promoter. As a proof of principle, we inserted three different promoters into three plasmids in front of the BioBrick BBa_I746916 ([http://parts.igem.org/Part:BBa_I746908 BBa_I746916, Main Page]) which encodes superfolded GFP. Then, we transformed cells with these plasmids and let them grow in media with different pHs in order to check the expression. | | We decided to use pH as the specific trigger that activates the promoter. As a proof of principle, we inserted three different promoters into three plasmids in front of the BioBrick BBa_I746916 ([http://parts.igem.org/Part:BBa_I746908 BBa_I746916, Main Page]) which encodes superfolded GFP. Then, we transformed cells with these plasmids and let them grow in media with different pHs in order to check the expression. |
| | | | |
| Line 39: |
Line 39: |
| | [[Image: Team-EPFL-Lausanne PCR_1.1+1.2-I.jpg|thumb|200px|left|Figure 7: 0.8% Gel, PCRs of the Hya and the Cad promoter]] | | [[Image: Team-EPFL-Lausanne PCR_1.1+1.2-I.jpg|thumb|200px|left|Figure 7: 0.8% Gel, PCRs of the Hya and the Cad promoter]] |
| | [[Image: Team-EPFL-Lausanne PCR_1.2-I.jpg|thumb|200px|left|Figure 8: 2% Gel, PCR of the constitutive promoter BBa_J23119]] | | [[Image: Team-EPFL-Lausanne PCR_1.2-I.jpg|thumb|200px|left|Figure 8: 2% Gel, PCR of the constitutive promoter BBa_J23119]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
| | | | |
| Line 56: |
Line 56: |
| | [[Image: Team-EPFL-Lausanne 1.2_OD_Measurements.jpg|thumb|200px|left|Figure 10: OD measurements of Bacteria with the cad promoter in different media ]] | | [[Image: Team-EPFL-Lausanne 1.2_OD_Measurements.jpg|thumb|200px|left|Figure 10: OD measurements of Bacteria with the cad promoter in different media ]] |
| | [[Image: Team-EPFL-Lausanne 1.3_OD_Measurements.jpg|thumb|200px|left|Figure 11: OD measurements of Bacteria with the constitutive promoter in different media ]] | | [[Image: Team-EPFL-Lausanne 1.3_OD_Measurements.jpg|thumb|200px|left|Figure 11: OD measurements of Bacteria with the constitutive promoter in different media ]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
| | | | |
| Line 69: |
Line 69: |
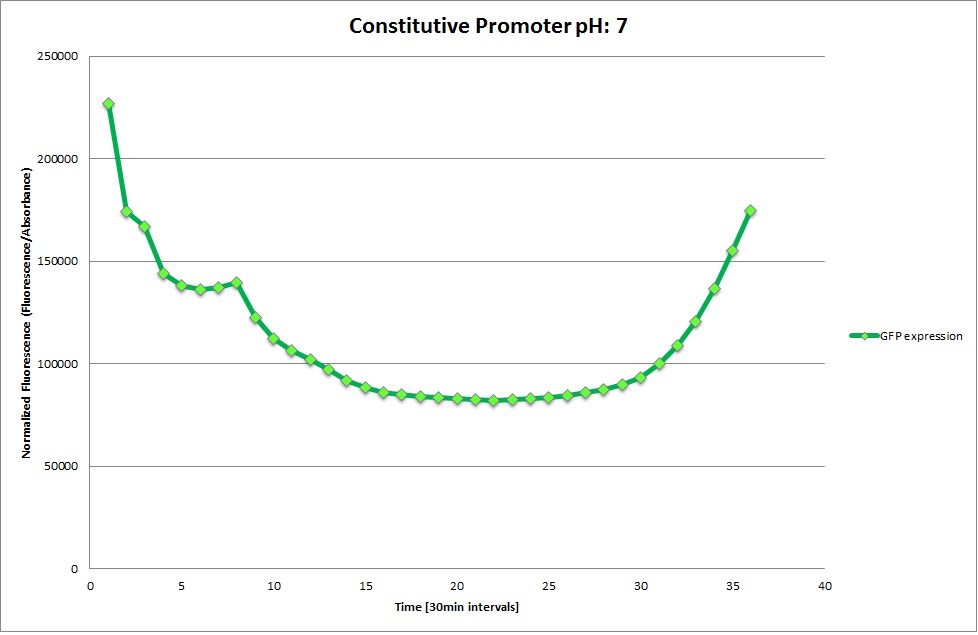
| | [[Image: Team-EPFL-Lausanne 1.1_Graph_pH3.jpg|thumb|200px|left|Figure 14: Normalized Fluorescence at pH 7.0 ]] | | [[Image: Team-EPFL-Lausanne 1.1_Graph_pH3.jpg|thumb|200px|left|Figure 14: Normalized Fluorescence at pH 7.0 ]] |
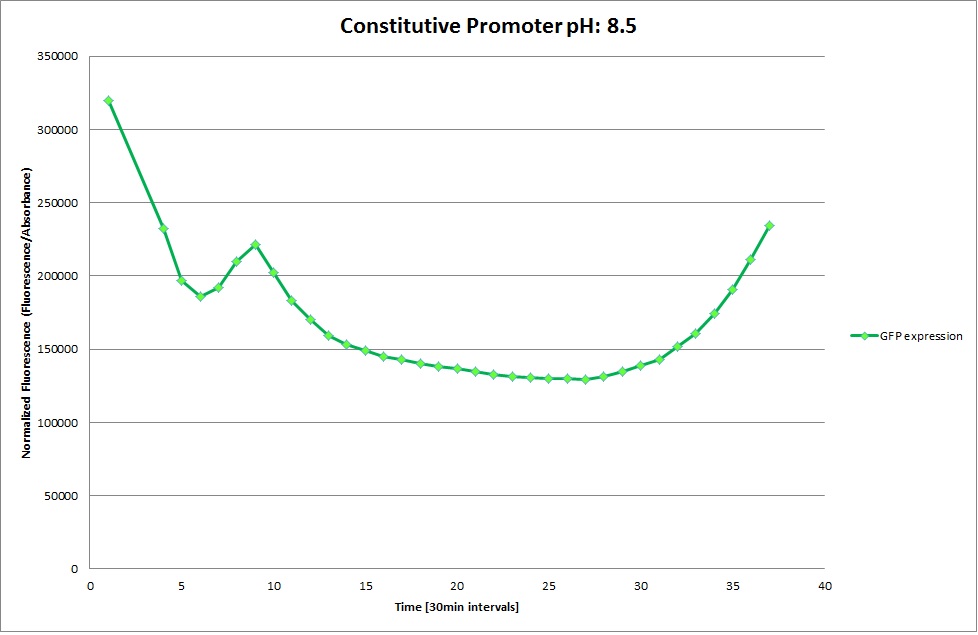
| | [[Image: Team-EPFL-Lausanne 1.1_Graph_pH4.jpg|thumb|200px|left|Figure 15: Normalized Fluorescence at pH 8.5 ]] | | [[Image: Team-EPFL-Lausanne 1.1_Graph_pH4.jpg|thumb|200px|left|Figure 15: Normalized Fluorescence at pH 8.5 ]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
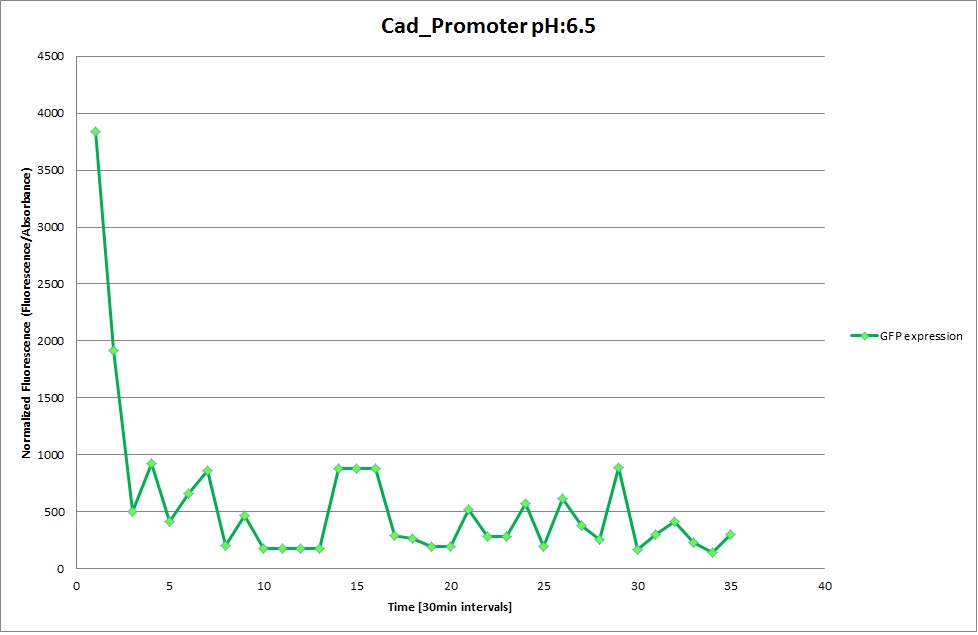
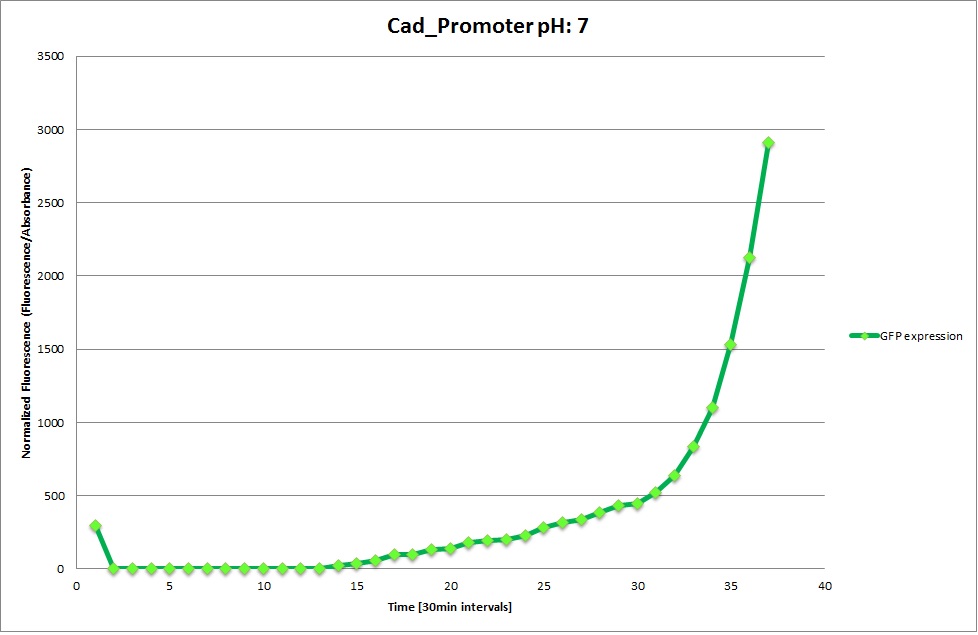
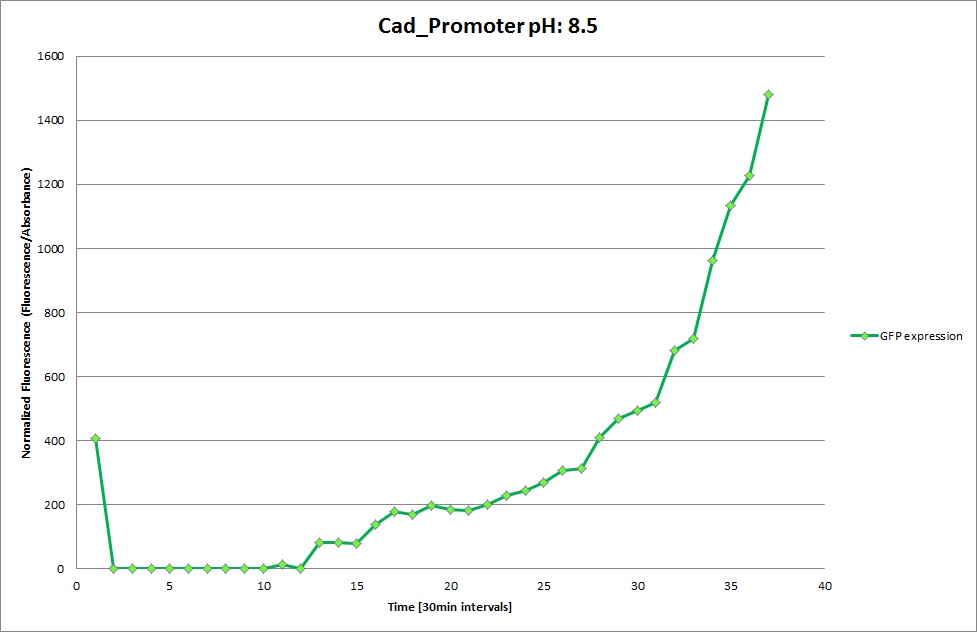
| | '''Cad Promoter''' | | '''Cad Promoter''' |
| Line 76: |
Line 76: |
| | [[Image: Team-EPFL-Lausanne 1.2_Graph_pH3.jpg|thumb|200px|left|Figure 18: Normalized Fluorescence at pH 7.0 ]] | | [[Image: Team-EPFL-Lausanne 1.2_Graph_pH3.jpg|thumb|200px|left|Figure 18: Normalized Fluorescence at pH 7.0 ]] |
| | [[Image: Team-EPFL-Lausanne 1.2_Graph_pH4.jpg|thumb|200px|left|Figure 19: Normalized Fluorescence at pH 8.5 ]] | | [[Image: Team-EPFL-Lausanne 1.2_Graph_pH4.jpg|thumb|200px|left|Figure 19: Normalized Fluorescence at pH 8.5 ]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
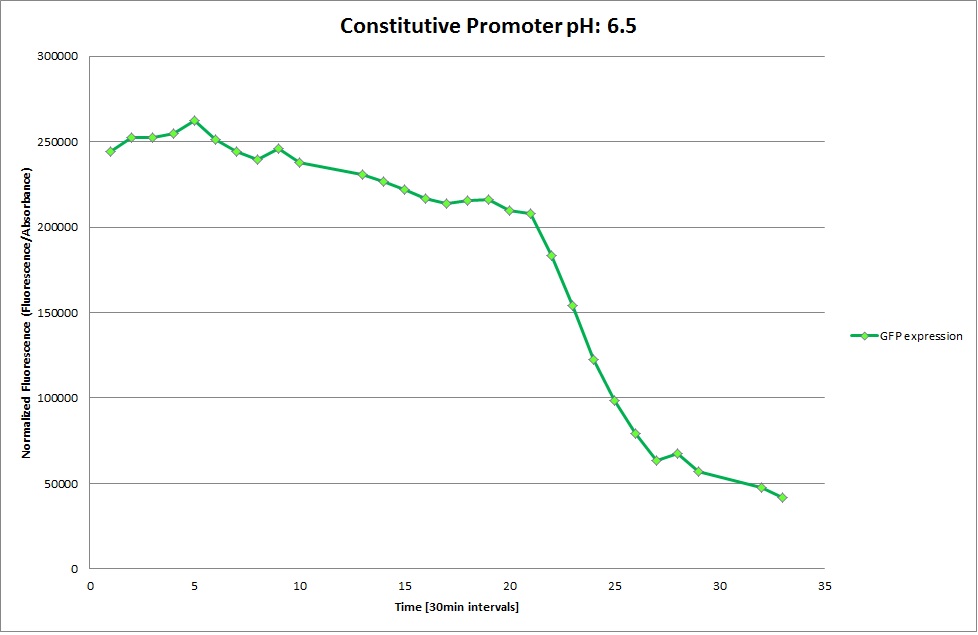
| | '''Constitutive Promoter''' | | '''Constitutive Promoter''' |
| Line 83: |
Line 83: |
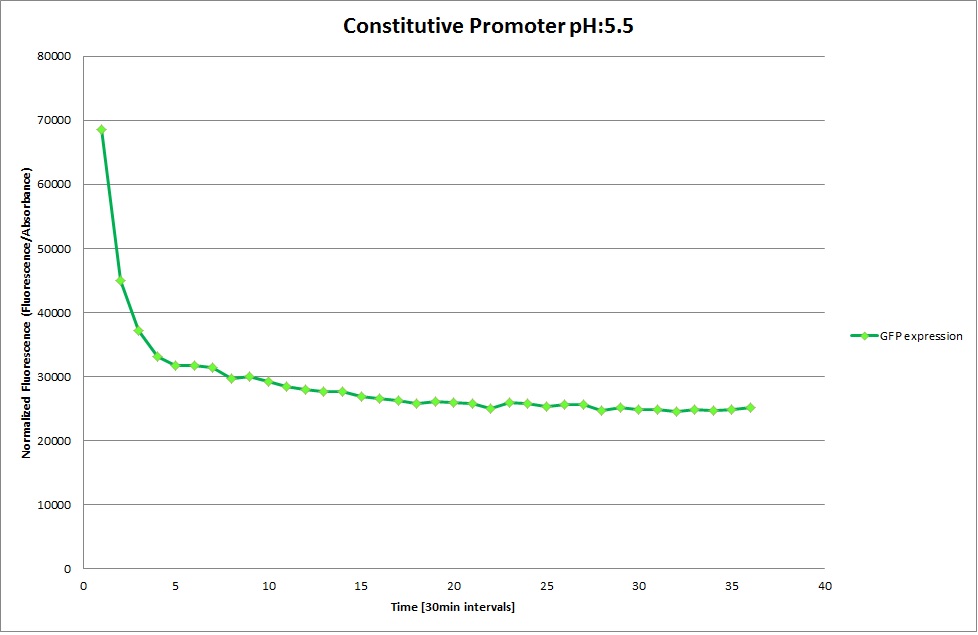
| | [[Image: Team-EPFL-Lausanne 1.3_Graph_pH3.jpg|thumb|200px|left|Figure 22: Normalized Fluorescence at pH 7.0 ]] | | [[Image: Team-EPFL-Lausanne 1.3_Graph_pH3.jpg|thumb|200px|left|Figure 22: Normalized Fluorescence at pH 7.0 ]] |
| | [[Image: Team-EPFL-Lausanne 1.3_Graph_pH4.jpg|thumb|200px|left|Figure 23: Normalized Fluorescence at pH 8.5 ]] | | [[Image: Team-EPFL-Lausanne 1.3_Graph_pH4.jpg|thumb|200px|left|Figure 23: Normalized Fluorescence at pH 8.5 ]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
| | ==Microscopy== | | ==Microscopy== |
| Line 92: |
Line 92: |
| | [[Image: Team-EPFL-Lausanne 1.1_MS_HEPES.jpg|thumb|200px|left|Figure 25: FRAC_image, exposure: 400ms, Multiplier 1, pH 8.5 ]] | | [[Image: Team-EPFL-Lausanne 1.1_MS_HEPES.jpg|thumb|200px|left|Figure 25: FRAC_image, exposure: 400ms, Multiplier 1, pH 8.5 ]] |
| | [[Image: Team-EPFL-Lausanne 1.1_MS_WATER.jpg|thumb|200px|left|Figure 26: FRAC_image, exposure: 550ms, Multiplier 1, pH 7 ]] | | [[Image: Team-EPFL-Lausanne 1.1_MS_WATER.jpg|thumb|200px|left|Figure 26: FRAC_image, exposure: 550ms, Multiplier 1, pH 7 ]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
| | '''Cad Promoter''' | | '''Cad Promoter''' |
| Line 98: |
Line 98: |
| | [[Image: Team-EPFL-Lausanne 1.2_MS_HEPES.jpg|thumb|200px|left|Figure 28: FRAC_image, exposure: 400ms, Multiplier 50, pH 8.5 ]] | | [[Image: Team-EPFL-Lausanne 1.2_MS_HEPES.jpg|thumb|200px|left|Figure 28: FRAC_image, exposure: 400ms, Multiplier 50, pH 8.5 ]] |
| | [[Image: Team-EPFL-Lausanne 1.2_MS_WATER.jpg|thumb|200px|left|Figure 29: FRAC_image, exposure: 400ms, Multiplier 50, pH 7 ]] | | [[Image: Team-EPFL-Lausanne 1.2_MS_WATER.jpg|thumb|200px|left|Figure 29: FRAC_image, exposure: 400ms, Multiplier 50, pH 7 ]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
| | '''Constitutive Promoter (Positive Control)''' | | '''Constitutive Promoter (Positive Control)''' |
| Line 104: |
Line 104: |
| | [[Image: Team-EPFL-Lausanne 1.3_MS_HEPES.jpg|thumb|200px|left|Figure 31: FRAC_image, exposure: 400ms, Multiplier 1, pH 8.5 ]] | | [[Image: Team-EPFL-Lausanne 1.3_MS_HEPES.jpg|thumb|200px|left|Figure 31: FRAC_image, exposure: 400ms, Multiplier 1, pH 8.5 ]] |
| | [[Image: Team-EPFL-Lausanne 1.3_MS_WATER.jpg|thumb|200px|left|Figure 32: FRAC_image, exposure: 400ms, Multiplier 1, pH 7 ]] | | [[Image: Team-EPFL-Lausanne 1.3_MS_WATER.jpg|thumb|200px|left|Figure 32: FRAC_image, exposure: 400ms, Multiplier 1, pH 7 ]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | ''Note''<BR> | | ''Note''<BR> |
| | We overexposed some of the images to be sure that there was no fluorescence in the bacteria in these media. | | We overexposed some of the images to be sure that there was no fluorescence in the bacteria in these media. |
| Line 112: |
Line 112: |
| | | | |
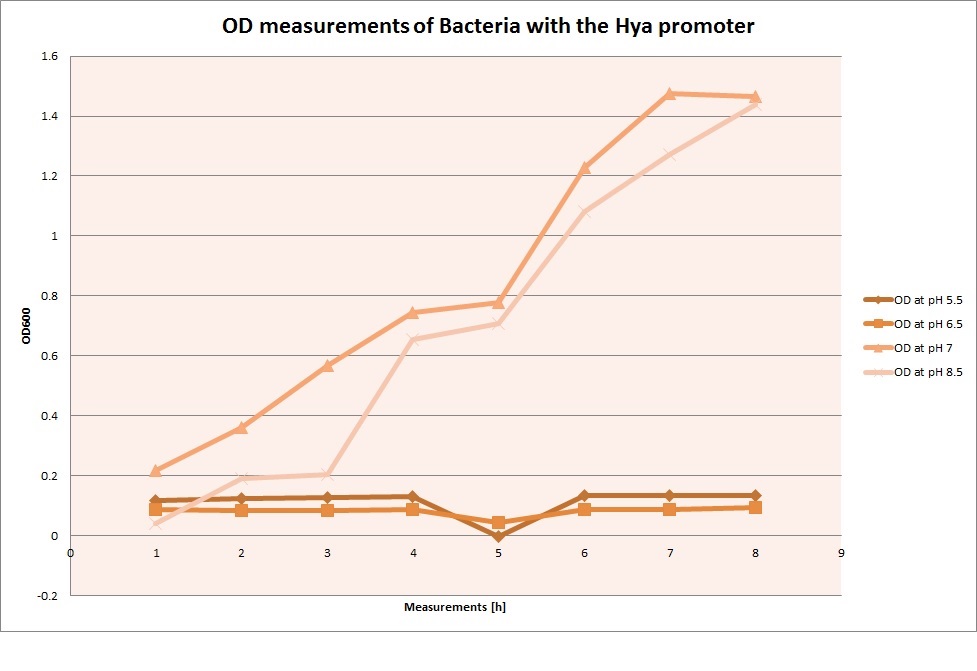
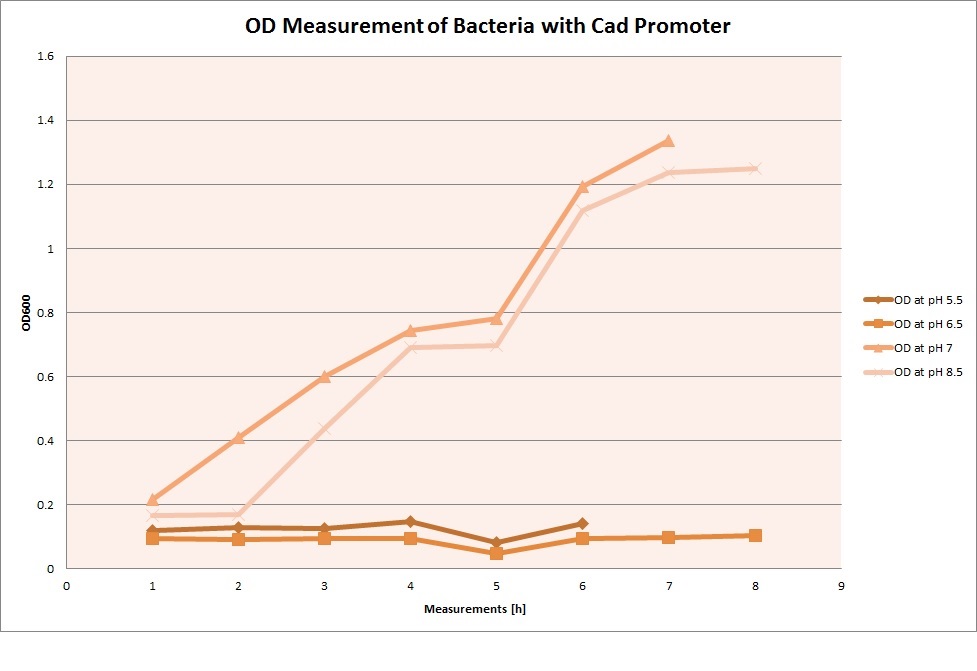
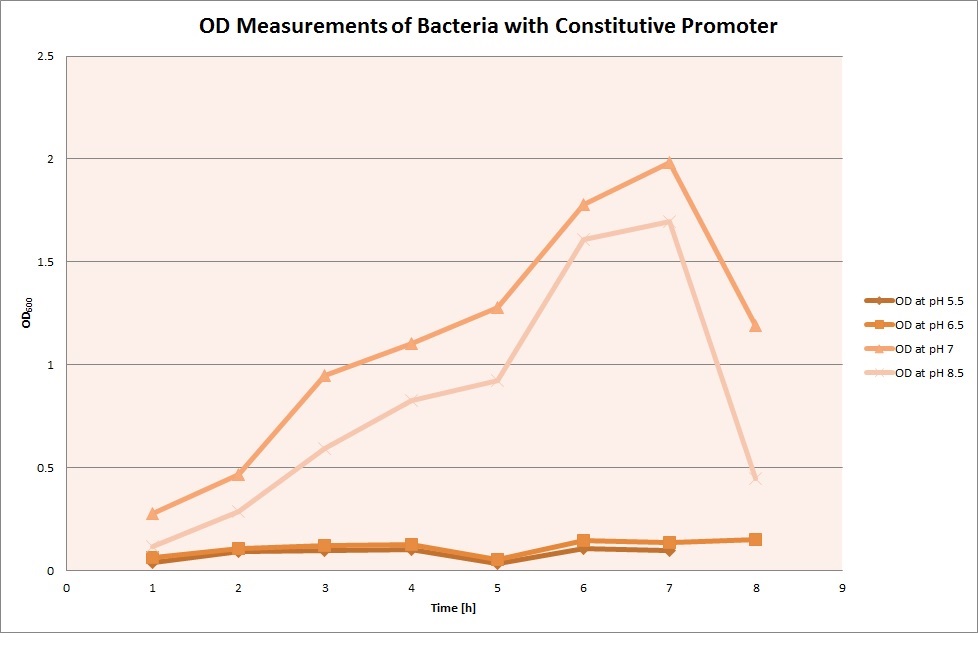
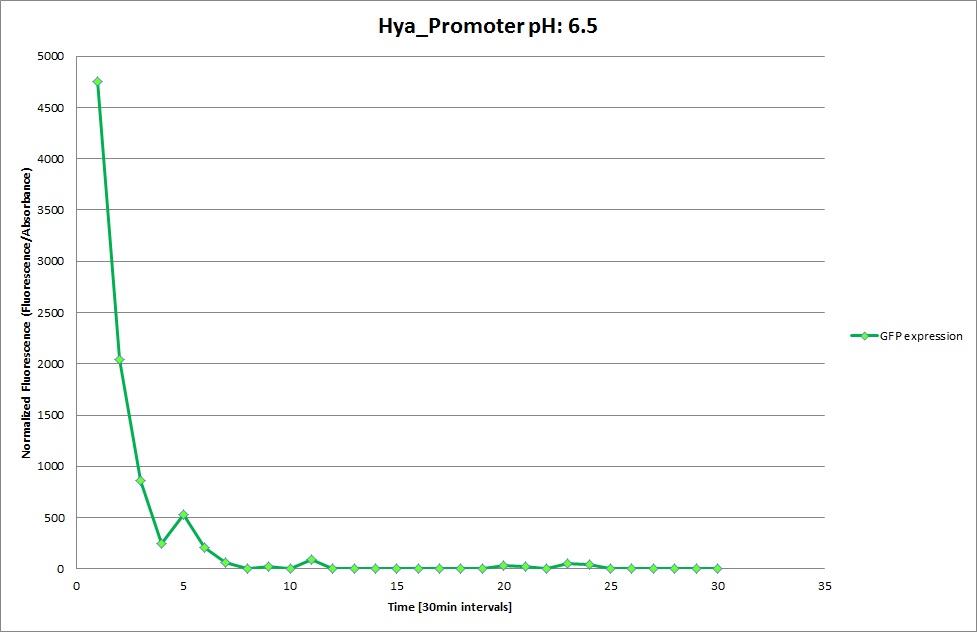
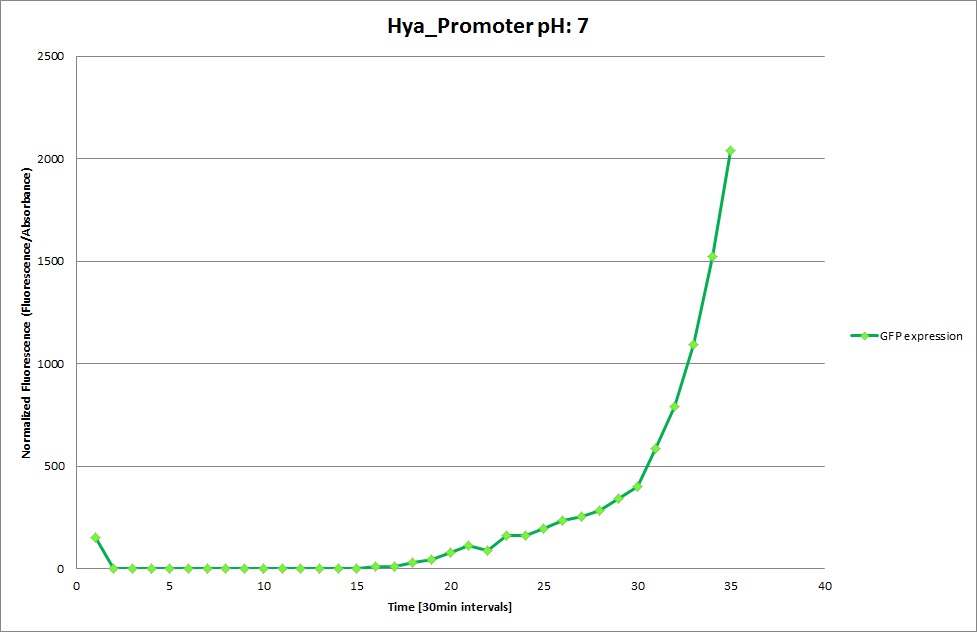
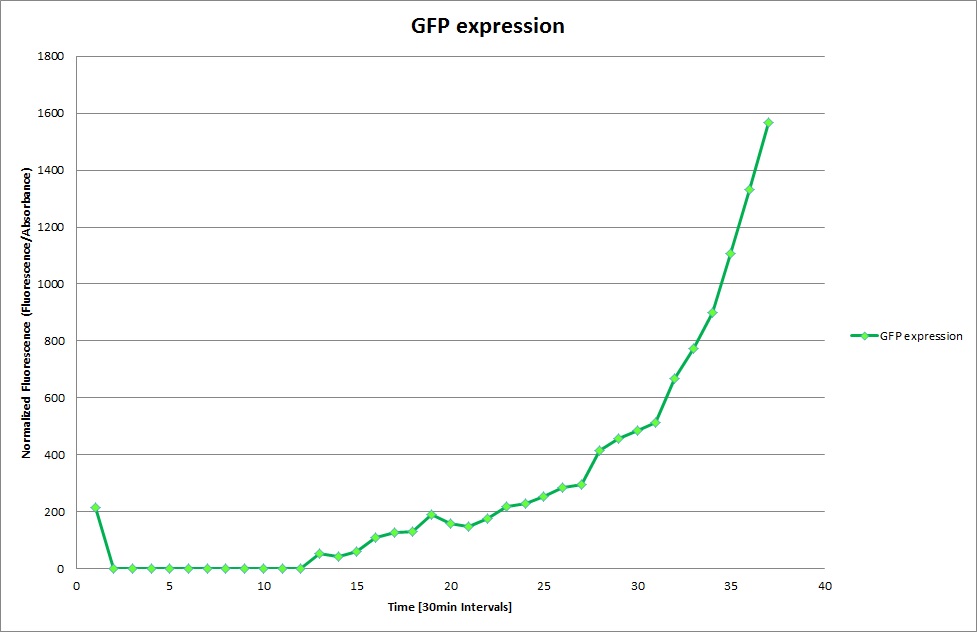
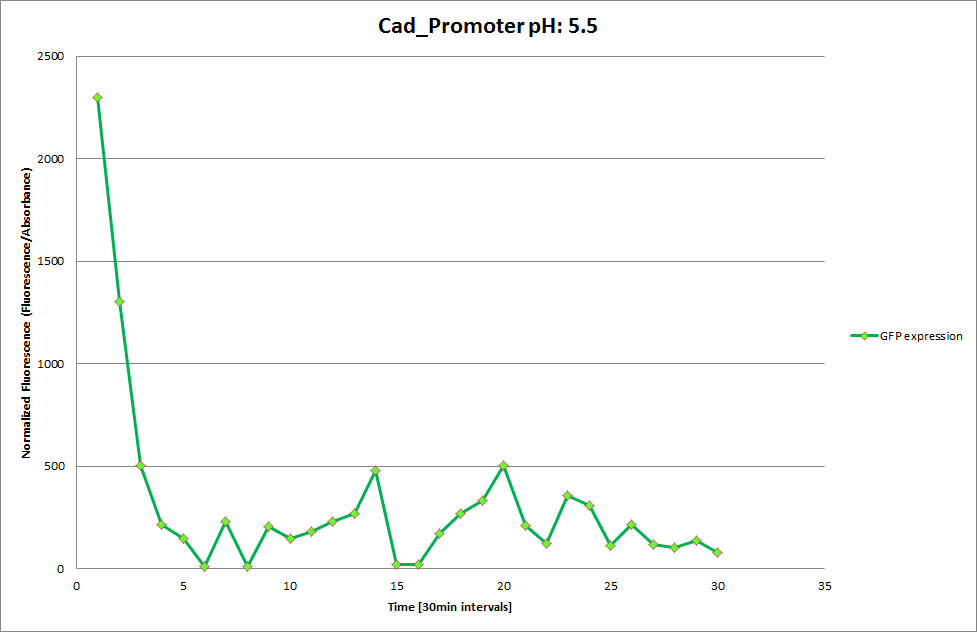
| | The promoters Hya and Cad were supposed to initiate transcription upon external acidification, which would then cause the bacteria to express superfolded GFP and turn bright green. The biobrick BBa_J23119, being a constitutive promoter, would turn the bacteria green independently of their medium. | | The promoters Hya and Cad were supposed to initiate transcription upon external acidification, which would then cause the bacteria to express superfolded GFP and turn bright green. The biobrick BBa_J23119, being a constitutive promoter, would turn the bacteria green independently of their medium. |
| - | For our project, the promoters were inserted in front of a gelatinase so that the latter would be expressed upon arrival in an acidic medium. The bacterium would then secrete the gelatinase causing the degradation of the naonoparticle and the release of its contents. | + | For our project, the promoters were inserted in front of a gelatinase so that the later would be expressed upon arrival in an acidic medium. The bacterium would then secrete the gelatinase causing the degradation of the nanoparticle and the release of its contents. |
| | | | |
| | ==Results== | | ==Results== |
| Line 127: |
Line 127: |
| | <BR> | | <BR> |
| | '''Conclusion'''<BR> | | '''Conclusion'''<BR> |
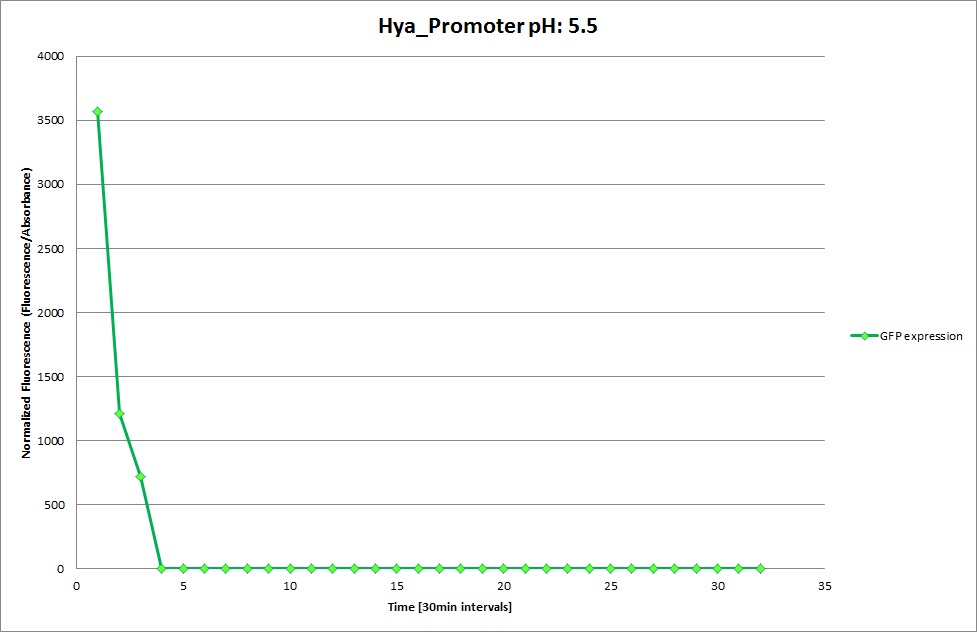
| - | Thus we can conclude that the hya promoter and the cad promoter work, in the sense that they in fact induce expression of GFP. But from our data, we were not able to conclusively say that they are inducible by low pH. | + | Thus, we can conclude that the hya promoter and the cad promoter work, in the sense that they in fact induce expression of GFP. But from our data, we were not able to conclusively say that they are inducible by low pH. |
| | Nevertheless, we were able to show that they are weak promoters that can be used to express a protein that should be present at low concentrations. | | Nevertheless, we were able to show that they are weak promoters that can be used to express a protein that should be present at low concentrations. |
| | | | |
| | '''With respect to our project''' <BR> | | '''With respect to our project''' <BR> |
| - | In order to be sure that hya and cad are induced only at low pH, we would need to do further testing. As soon as their inducibility would be confirmed, we would insert them by gibson assemly in front of either GelE gelatinase or MMP2 gelatinase. Upon arrival in a medium with pH bellow 7 the enzyme would be synthetised and released. | + | In order to be sure that hya and cad are induced only at low pH, we would need to do further testing. As soon as their inducibility would be confirmed, we would insert them by gibson assembly in front of either GelE gelatinase or MMP2 gelatinase. Upon arrival in a medium with pH below 7 the enzyme would be synthesized and released. |
| | | | |
| | | | |
| Line 144: |
Line 144: |
| | | | |
| | [http://jb.asm.org/content/173/15/4851.abstract?sid=2a119bc7-0c26-4543-a671-d3a50521253f [2]] Journal of Bacteriology <BR> | | [http://jb.asm.org/content/173/15/4851.abstract?sid=2a119bc7-0c26-4543-a671-d3a50521253f [2]] Journal of Bacteriology <BR> |
| - | Mutational analysis and charecterization of the Escherichia coli hya operin, which encodes [NiFe] hydrogenase1 <BR> | + | Mutational analysis and characterization of the Escherichia coli hya operin, which encodes [NiFe] hydrogenase1 <BR> |
| | N K Menon, J Robbins, J C Wendt, K T Shanmuagam and A E Przybyla <BR> J. Bacteriol 1991 <BR> | | N K Menon, J Robbins, J C Wendt, K T Shanmuagam and A E Przybyla <BR> J. Bacteriol 1991 <BR> |
| | | | |
| Line 157: |
Line 157: |
| | J. Bacteriol 1992 <BR> | | J. Bacteriol 1992 <BR> |
| | | | |
| - | ==Effecting== | + | =='''Effecting'''== |
| | | | |
| | ==Overview== | | ==Overview== |
| | | | |
| - | The goal of the effecting part of the project was to build a plasmid containing an arabinose-inducible promoter driving a tagged enzyme able to cleave gelatine, the polymer used to build our nanoparticles. We chose three different enzymes that would be inserted in part BBa_I746908(a pBAD promoter driving superfolder GFP, [http://parts.igem.org/Part:BBa_I746908 [registry page]]) either between the promoter and GFP reporter or replacing the GFP, since having such a reporter at the end of a protein sometimes causes folding troubles. All of the constructs were designed to have a His tag in addition so we could extract and purify our expressed protein. | + | The goal of the effecting part was to build a plasmid containing an arabinose-inducible promoter driving a tagged enzyme able to cleave gelatin, the polymer used to build our nanoparticles. We chose three different enzymes that were inserted into part BBa_I746908([http://parts.igem.org/Part:BBa_I746908 [BBa_I746908, Main Page]) either between the promoter and GFP or replacing the GFP. All of the constructs were designed to have a His tag at the beginning of the protein so we could extract and purify our expressed protein. |
| | <br> | | <br> |
| - | The final desired result was bacteria transformed with the plasmid who secreted our gelatine-degrading protein upon the addition of arabinose to the culture medium and/or glowed green (in the case where we left GFP in the plasmid)
| + | We would then transform cells with our construct, make them express the gelatinase by inducing the promoter and then isolate and purify the protein. |
| | | | |
| - | ==Strategy==
| |
| - |
| |
| - | The strategy we decided to go for to build our plasmids was the Gibson assembly technique. To perform this technique, complementary overlaps must first be added at the 5' and 3' ends of both our insert (GelE, MMP2 or MMP9 in this case) and backbone (BBa_I746908). These complemetary overlaps then bind together, inserting the insert at the desired position in the backbone.
| |
| - | To do so, we planned to do PCR reactions whose primers contained: the overlap, a Histag and a part of the protein CDS for the inserts and the overlap and a part of the backbone CDS for the backbone. The final plasmids would have pBAD/araC-START-Histag-ProteinCDS-STOP or pBAD/araC-START-Histag-ProteinCDS-linker-GFP-STOP as a part.
| |
| - | The primers for the constructs that worked are listed in the "Reference & Information" paragraph.
| |
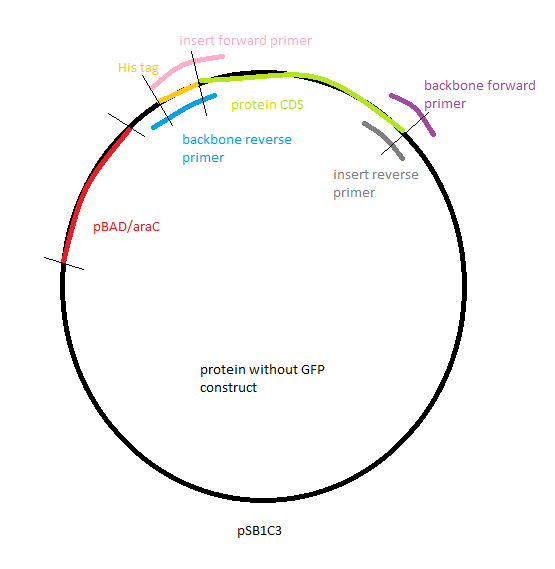
| | [[Image: protein wo GFP construct.PNG|thumb|200px|left|Figure 33: Gibson assembly: Protein without GFP]] | | [[Image: protein wo GFP construct.PNG|thumb|200px|left|Figure 33: Gibson assembly: Protein without GFP]] |
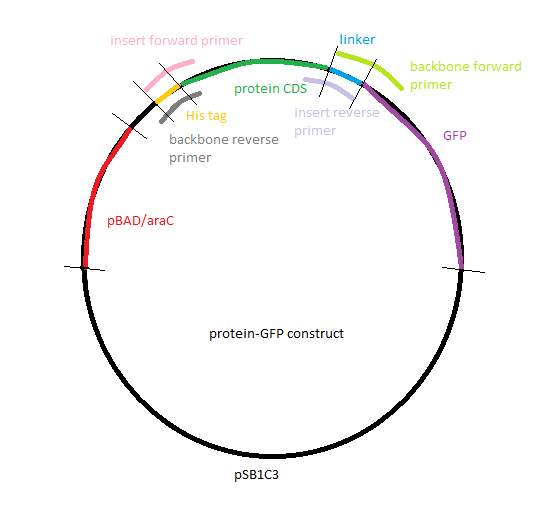
| | [[Image: GFP construct.PNG|thumb|200px|left|Figure 34: Gibson assembly: Protein with GFP]] | | [[Image: GFP construct.PNG|thumb|200px|left|Figure 34: Gibson assembly: Protein with GFP]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
| | ==Experiments== | | ==Experiments== |
| | | | |
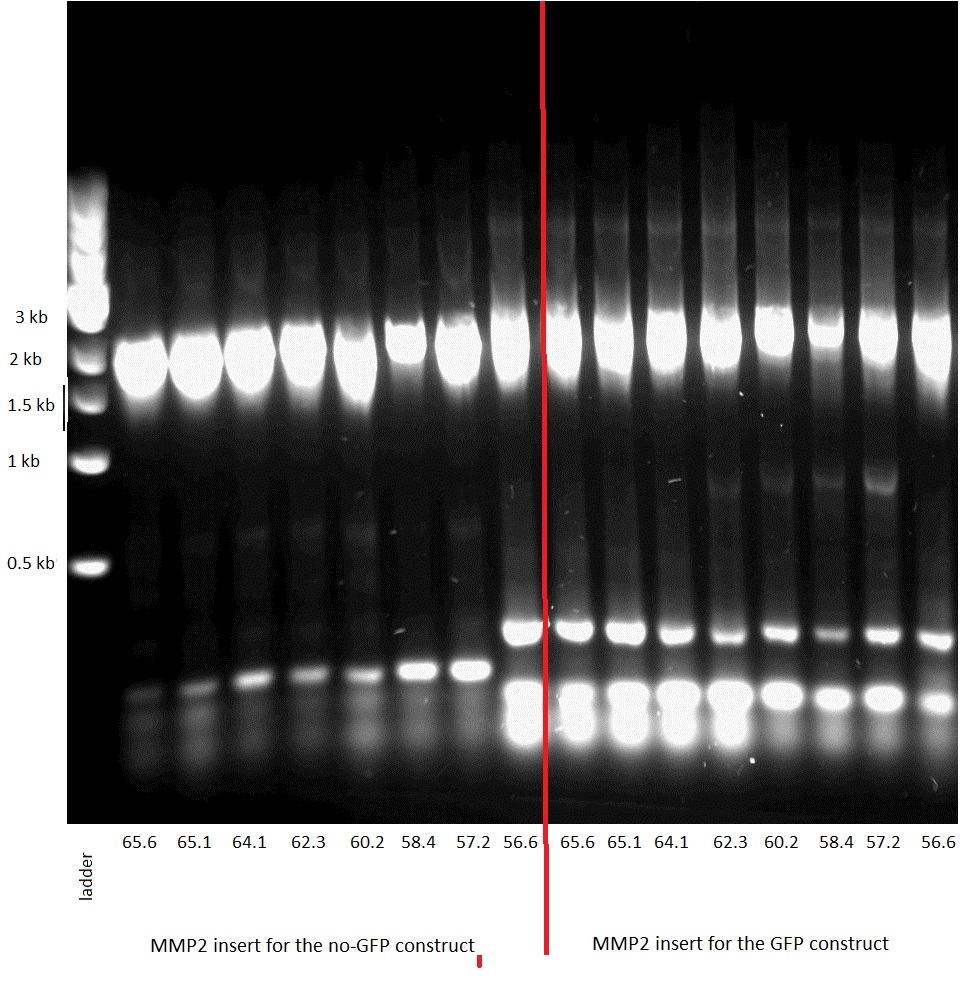
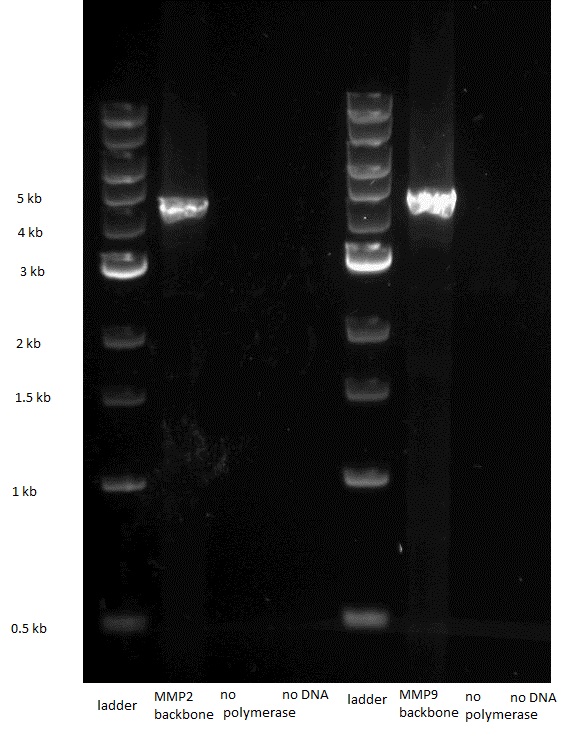
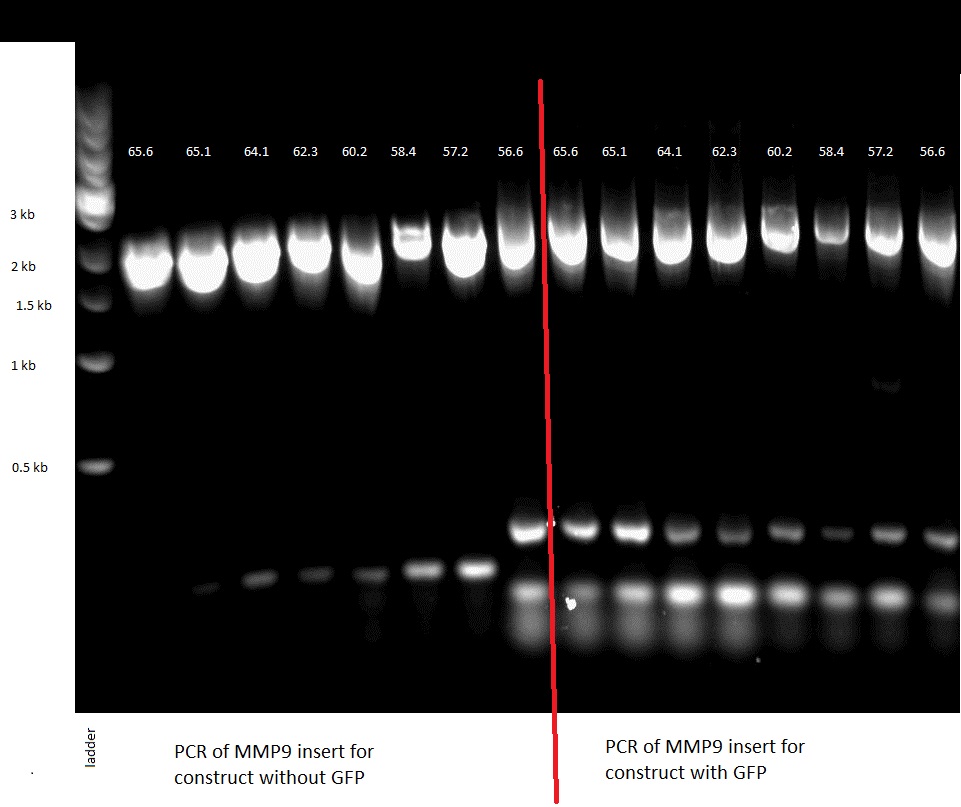
| - | The three different enzymes that we chose to use were gelatinase E (gelE) from Gram positive bacterium E. faecalis ([http://www.example.com NCBI page]), metalloprotease 2 (MMP2) from H. sapiens ([http://plasmid.med.harvard.edu/PLASMID/GetCloneDetail.do?cloneid=2849&species=Homo%20sapiens reference]) and metalloprotease 9 (MMP9) from M. musculus. We ordered the genomic DNA of E. faecalis as well as two plasmids containing the CDSs of MMP2 and MMP9 from plasmid.med.harvard.edu. | + | The three different enzymes that we chose to use were gelatinase E (gelE) from Gram positive bacterium E.faecalis , metalloprotease 2 (MMP2) from H. sapiens [http://plasmid.med.harvard.edu/PLASMID/GetCloneDetail.do?cloneid=2849&species=Homo%20sapiens [1]] and metalloprotease 9 (MMP9) from M. musculus. We ordered the genomic DNA of E.faecalis as well as two plasmids containing the CDSs of MMP2 and MMP9 from plasmid.med.harvard.edu. |
| | | | |
| - | '''PCR''' <BR>
| + | ==PCR== |
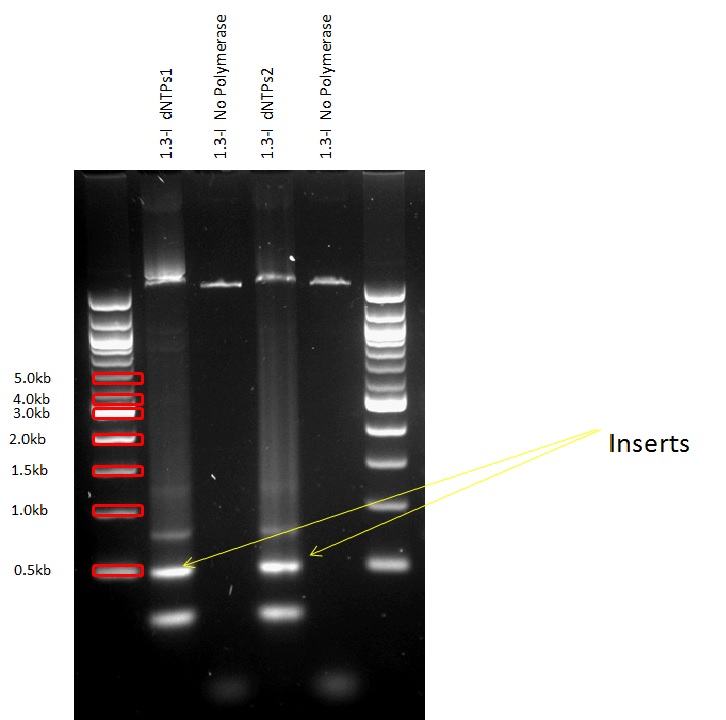
| - | We first amplified the CDS of our three proteins by PCR, using the primers described in the "Strategy" paragraph. The expected weights for our products were: 1,5kb for the GelE insert,2kb for the MMP2 and MMP9 inserts, 3.2 for the backbone without GFP and 4.2kb for the backbone still containing GFP. The PCRs for the backbones having the GFP removed did unfortunately not work, so we had to leave the three constructs that needed them behind. | + | We first amplified the CDS of our three proteins by PCR. Because we had introduced a frame shift during the PCR due to our primers, we had constructs that had a stop codon in front of GFP. Thus we decided to continue with these construct knowing that we could still purify the protein because of the His-Tag we added. |
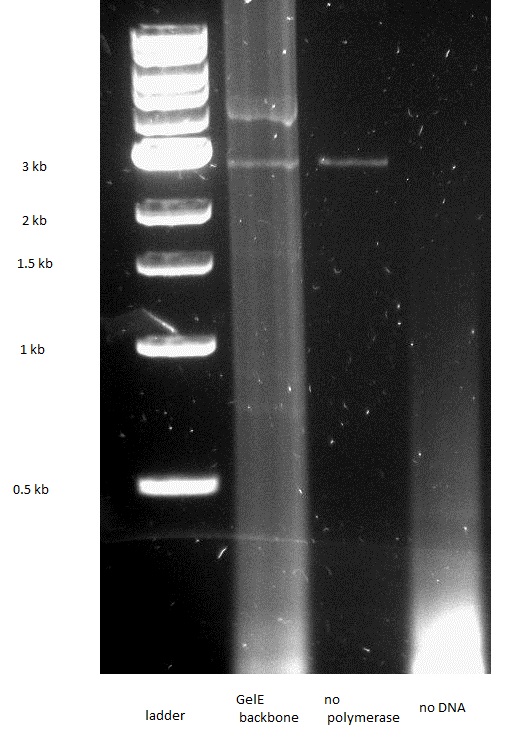
| - | [[Image: GelE insert PCR.JPEG|thumb|200px|left|Figure 35: PCR: GelE insert]] | + | [[Image: GelE insert PCR.JPEG|thumb|200px|left|Figure 35: 0.8% Gel, PCR of the GelE insert]] |
| - | [[Image: GelE backbone PCR.JPEG|thumb|200px|left|Figure 36: PCR: GelE backbone]] | + | [[Image: GelE backbone PCR.JPEG|thumb|200px|left|Figure 36: 0.8% Gel, PCR of the GelE backbone]] |
| - | [[Image: MMP2 insert PCR.JPEG|thumb|200px|left|Figure 37: PCR: MMP2 insert]] | + | [[Image: MMP2 insert PCR.JPEG|thumb|200px|left|Figure 37: 0.8% Gel, PCR of the MMP2 insert]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| - | [[Image: MMP2&MMP9 backbone PCR.JPEG|thumb|200px|left|Figure 38: PCR: MMP2 & MMP9 backbones]] | + | [[Image: MMP2&MMP9 backbone PCR.JPEG|thumb|200px|left|Figure 38: 0.8% Gel,PCR of the MMP2 & MMP9 backbones]] |
| - | [[Image: MMP9 insert PCR.JPEG|thumb|200px|left|Figure 39: PCR: MMP9 insert]] | + | [[Image: MMP9 insert PCR.JPEG|thumb|200px|left|Figure 39: 0.8% Gel, PCR of teh MMP9 insert]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| - | Then, we proceeded to a PCR purification.
| + | We then purified the PCR products. |
| | + | |
| | + | ==Gibson Assembly== |
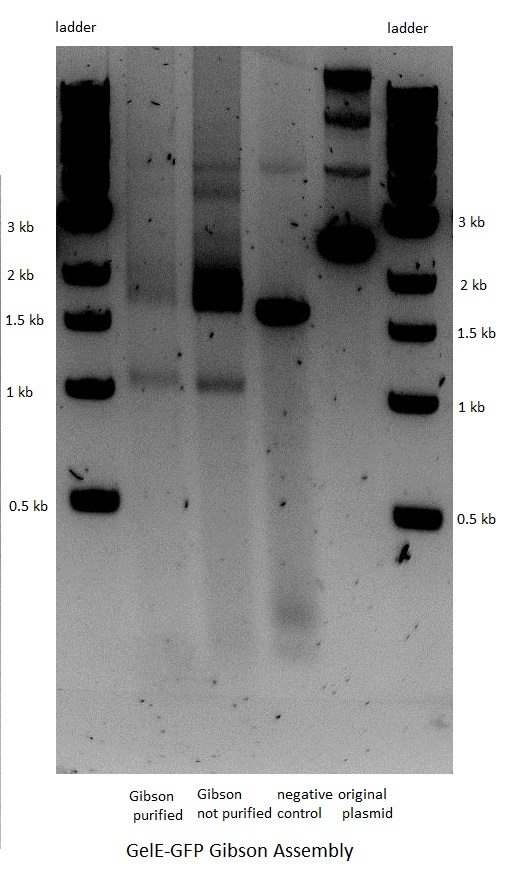
| | + | The next step was the Gibson assembly reaction which would put together the inserts and their corresponding backbones. The reaction was performed only for the constructs that should have GFP as a reporter but had a stop in front of the latter because of the above mentioned frame shift. The three other constructs that were not supposed to have GFP after the gelatinase gene were not done, as the result would have been the same. <BR> |
| | + | The assembly worked for both the GelE and MMP2 constructs, but not for MMP9.<BR> |
| | | | |
| - | '''Gibson Assembly'''<BR>
| |
| - | The next step was the Gibson assembly reaction which would put together the inserts and their corresponding backbones, thus assembling our constructs. The reaction was performed only for the constructs that were built to have the GFP reporter in the plasmid, the three other constructs being left aside due to a PCR issue. <BR>
| |
| - | The assembly worked for both the GelE-GFP and MMP2-GFP constructs, but not for MMP9.<BR>
| |
| - | The expected weights of the constructs were of 5.7kb for the GelE-GFP and 6.2kb for the MMP2-GFP. The band at 6 kb is clear for the MMP2-GFP construct, but the one at 5.7 isn't really visible for the GelE-GFP construct.We would be able to check for the presence of both constructs at the next step, after the minipreps were done.
| |
| | [[Image: GelE Gibson.JPEG|thumb|200px|left|Figure 40: Gibson Assembly: GelE-GFP construct]] | | [[Image: GelE Gibson.JPEG|thumb|200px|left|Figure 40: Gibson Assembly: GelE-GFP construct]] |
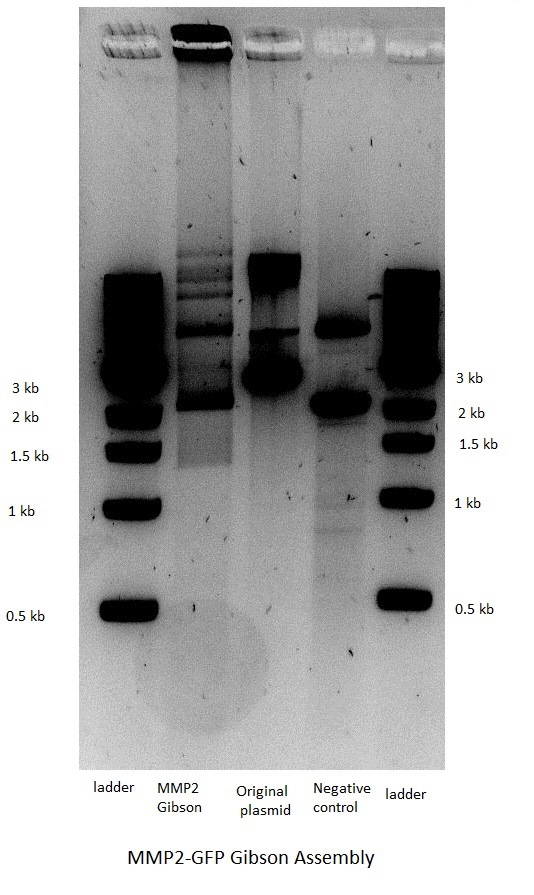
| | [[Image: MMP2 Gibson.JPEG|thumb|200px|left|Figure 41: Gibson Assembly: MMP2-GFP construct]] | | [[Image: MMP2 Gibson.JPEG|thumb|200px|left|Figure 41: Gibson Assembly: MMP2-GFP construct]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | | | |
| - | '''Transformation and sequencing'''<BR>
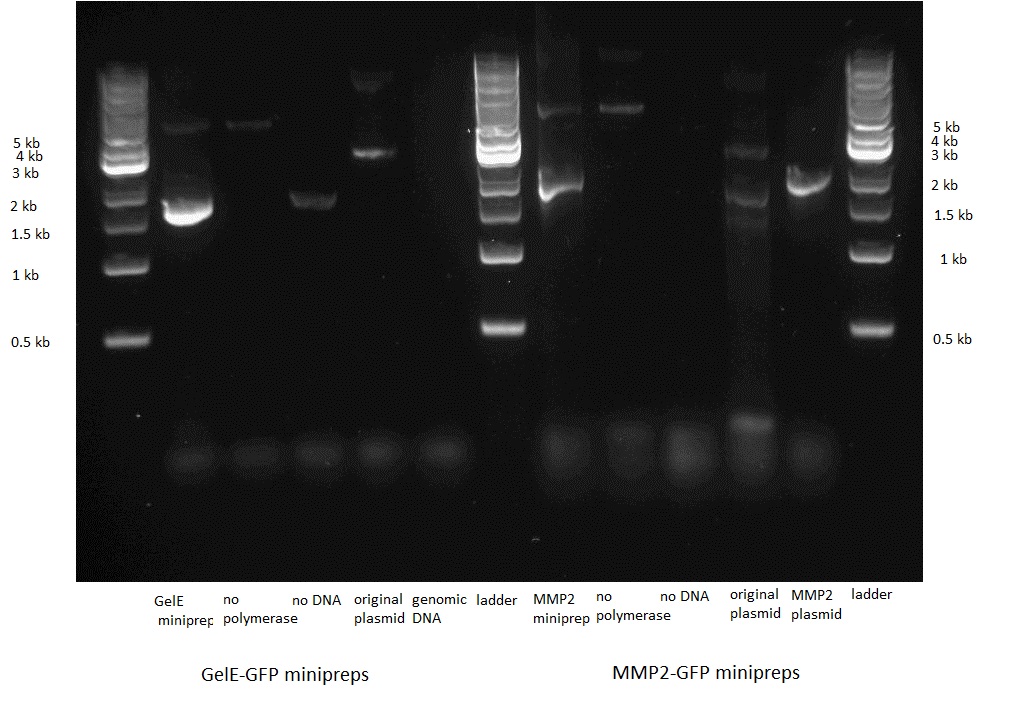
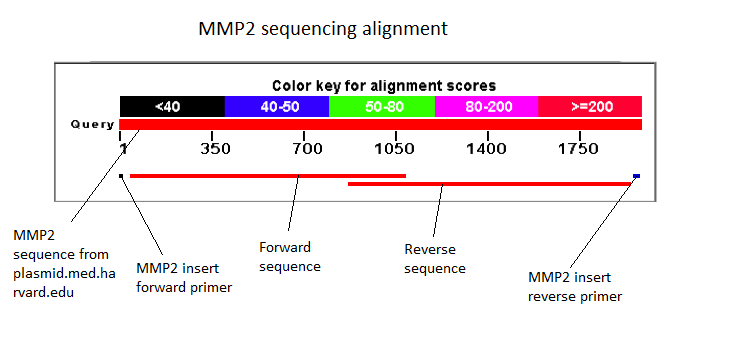
| + | ==Transformation, control PCR and sequencing== |
| - | We then transformed bacteria with the plasmids, isolated them and sent the minipreps for sequencing. The sequencing revealed the presence of an unexpected STOP codon just before the start of the GFP sequence. We still performed the glowing assay by adding 50ul of 20% arabinose to the 5ml culture medium in which we had grown the transformed bacteria overnight but the result came out negative.<BR> | + | We then transformed bacteria with the two constructs. Before sending them for sequencing we did a control PCR on each using primers that were specific to the insert, i.e. the GelE and the MMP2 gene.<BR> |
| - | The minipreps show the presence of the two expected constructs at 5.7kb and 6.2kb, repectively.
| + | [[Image: MMP2 GelE minipreps.JPEG|thumb|250px|center|Figure 42: Minipreps: GelE-GFP and MMP2-GFP constructs]]<BR><BR> |
| - | [[Image: MMP2 GelE minipreps.JPEG|thumb|200px|left|Figure 42: Minipreps: GelE-GFP and MMP2-GFP constructs]] | + | [[Image: Team-EPF-Lausanne-3.4_Seque_FV.jpg|thumb|300px|left|Figure 43: Sequence analysis of the GelE gene with a forward Primer]] |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | + | [[Image: Team-EPF-Lausanne-3.4_Seque_RV.jpg|thumb|300px|right|Figure 44: Sequence analysis of the GelE gene with a reverse Primer]] |
| | + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | + | [[Image: GelE sequence overlap.JPEG|thumb|200px|center|Figure 45: GelE sequencing overlaps]]<BR> |
| | + | [[Image: MMP2 sequence overlap.JPEG|thumb|200px|center|Figure 46: MMP2 sequencing overlaps]] |
| | + | [[Image: Team-EPF-Lausanne-5.4_Seque_FV.jpg|thumb|300px|left| Figure 47: Figure 43: Sequence analysis of the MMP2 gene with a forward Primer]] |
| | + | [[Image: Team-EPF-Lausanne-5.4_Seque_RV.jpg|thumb|300px|right|Figure 43: Sequence analysis of the MMP2 gene with a reverse Primer]] |
| | + | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> |
| | + | We also incubated the positive transformants with arabinose and looked at them under the microscope. As expected they did not express GFP. |
| | | | |
| | + | ==Western Blot== |
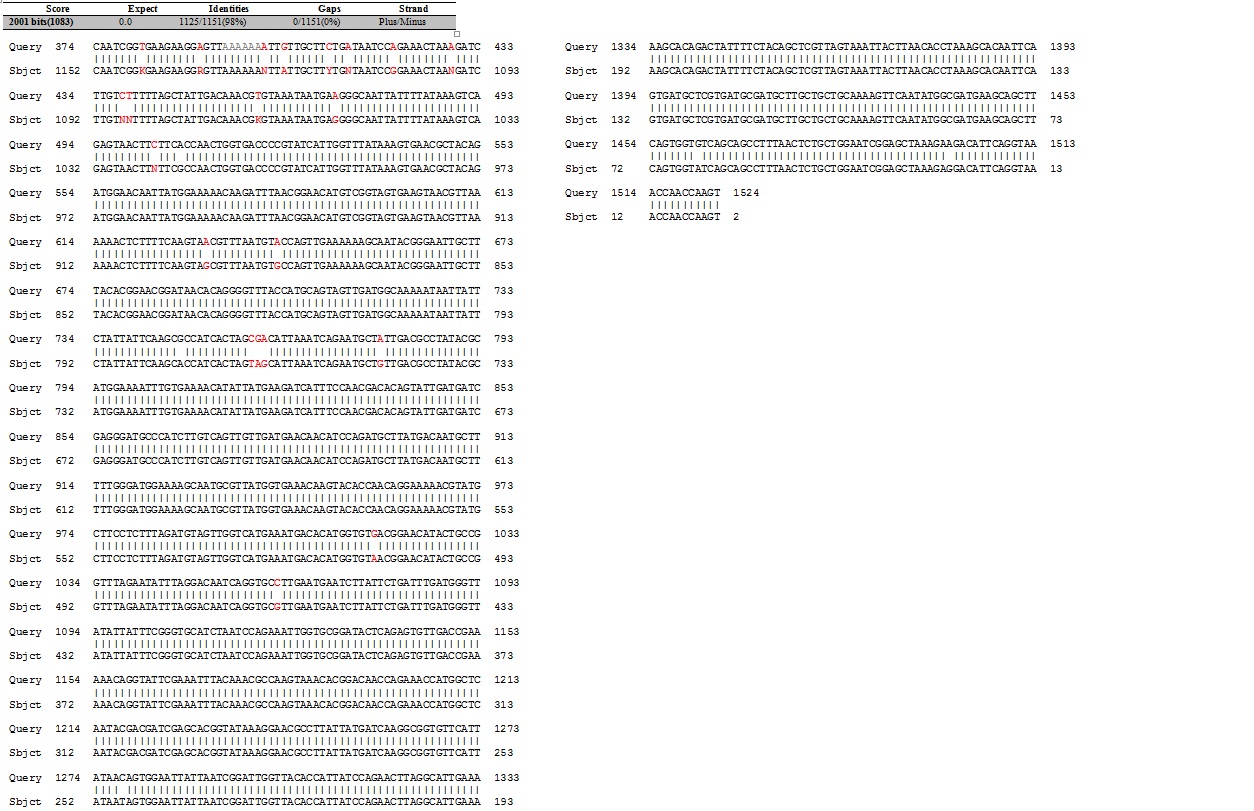
| | + | The next assay to test the expression and secretion of our proteins was a Western Blot, performed on the supernatant and cell lysate fractions of our liquid cell culture. The antibody used to detect our protein was an anti-His tag antibody, which was supposed to detect the His-tag that had been added to the CDS of our proteins. This assay unfortunately came out negative. We were unable to check for the presence of the His tag with the sequencing because the primers we used to do it did not initiate sequencing at the beginning of the protein as the sequencing starts a bit downstream of the primer binding site. The sequencing of the middle sequence showed a 97%-98% match between the insert and the reference sequence. |
| | | | |
| - | '''Western Blot'''<BR>
| + | <BR><BR> |
| - | The next assay to test the expression and secretion of our fusion proteins was a Western Blot, performed on the medium and cell lysate fractions of our liquid cell culture. The antibody used to detect our protein was an anti-His tag antibody, which was supposed to detect the his tag that had been added to the CDS of our fusion proteins. This assay unfortunately also came out negative. Also, we were unable to check for the presence of the His tag with the sequencing because the primers we used to do it were the same as for the Gibson. Thus, the sequencing starts a bit downstream of the primers, not sequencing them. However, their sequence was correct when we checked the order we had made.
| + | |
| - | [[Image: GelE sequence overlap.JPEG|thumb|200px|left|Figure 43: GelE sequencing overlaps]]
| + | |
| - | [[Image: MMP2 sequence overlap.JPEG|thumb|200px|left|Figure 44: MMP2 sequencing overlaps]]
| + | |
| - | <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR>
| + | |
| | | | |
| - | '''His-tag purification and SDS-PAGE'''<BR>
| + | ==Protein purification and SDS-PAGE== |
| - | A His tag purification method under native conditions using a Ni-NTA spin kit was performed. The native conditions were chosen because we wanted to perform a digestion assay with the purified protein on the nanoparticles to assess their digestion capacities. | + | A protein purification method under native conditions using a Ni-NTA spin kit was performed. The native conditions were chosen because we wanted to perform a digestion assay with the purified protein on the nanoparticles to assess their digestion capacities. |
| - | The lysates, flow-through, wash and final eluate (supposed to contain the purified protein) were kept and analysed with a NanoDrop before being used for an SDS-PAGE. The NanoDrop revealed that the eluates contained no protein, but that the wash fraction contained about 0.4 mg/ml of protein. The two possible reasons for this were: 1) the His tag was not properly added to the protein CDS (PCR mutation, ...) or 2) the His tag was hidden in the protein structure and could not bind to the column since the purification was done under native conditions. Therefore, the SDS-PAGE could reveal the presence of our protein by comparing the SDS-PAGE results of the protein from the arabinose-induced cell culture and those from the un-induced cells (negative control). | + | The lysates, flow-through, wash and final eluate (supposed to contain the purified protein) were kept and analyzed with a NanoDrop before being used for an SDS-PAGE. The NanoDrop revealed that the eluates contained no protein, but that the wash fraction contained about 0.4 mg/ml of protein. Possible reasons for this were: <BR> |
| - | Unfortunately, the experiment did not work due to a Coomassie stain issue: nothing could be visualized.
| + | 1) the His tag was not properly added to the protein CDS <BR> |
| | + | 2) the His tag was hidden in the protein structure and could not bind to the column since the purification was done under native conditions<BR> |
| | + | 3.) As the Kit was left at room temperature it is possible that the binding columns were defective <BR> |
| | + | When we did the SDS Page did not work due to a Coomassie stain issue. |
| | | | |
| | ==Discussion and Future== | | ==Discussion and Future== |
| | | | |
| - | We unfortuately could not come to a satisfying final result in the effecting part of the project because we could not prove that the plasmids actually conferred to the bacteria the ability to secrete a gelatine-degrading protein. We can later perform the digestion assay with the wash fraction of the Ni-NTA purification to see whether a gelatine-degradig protein is indeed present in it. Also, the next experiment would be to redo the SDS-PAGE on the remaining of the Ni-NTA purification. | + | We unfortunately could not come to a satisfying final result in the effecting part of the project because we could not prove that the plasmids actually conferred to the bacteria the ability to secrete a gelatin-degrading protein. We can later perform the digestion assay with the wash fraction of the Ni-NTA purification to see whether a gelatin-degrading protein is indeed present in it. Also, the next experiment would be to redo the SDS-PAGE on the remains of the Ni-NTA purification. |
| - |
| + | |
| - | ==References & Information==
| + | |
| - | | + | |
| - | GelE-GFP insert forward primer:5'-ATG CACCACCACCACCACCAC AAG GGA AAT AAA ATT TTA TAC CAT TTT A-3' <BR>
| + | |
| - | GelE-GFP insert reverse primer:5'-ACC ACC AGA TTG AAA ATA CAA ATT TTC ACC TTC ATT GAC CAG AAC AGA TTC-3' <BR>
| + | |
| - | BBa_I746908 backbone forward primer:5'-GGT GAA AAT TTG TAT TTT CAA TCT GGT GGT GAT GCG TAA AGG CGA AGA GCT-3' <BR>
| + | |
| - | BBa_I746908 backbone reverse primer:5'-ATT TCC CTT GTG GTG GTG GTG GTG GTG CAT TAG TAT TTC TCC TCT TTC TCT AGT AGC TAG-3' <BR><BR>
| + | |
| | | | |
| - | MMP2-GFP insert forward primer:5'-ATG CACCACCACCACCACCAC GAG GCG CTA ATG GCC C-3' <BR>
| + | To learn more about how we would continue, see [https://2013.igem.org/Team:EPF_Lausanne/Next_steps Next Steps] or [https://2013.igem.org/Team:EPF_Lausanne/Perspectives Perspectives]<BR> |
| - | MMP2-GFP insert reverse primer:5'-ACC ACC AGA TTG AAA ATA CAA ATT TTC ACC GCA GCC TAG CCA GTC GGA TTT GAT-3' <BR>
| + | |
| - | BBa_I746908 backbone forward primer:5'-GGT GAA AAT TTG TAT TTT CAA TCT GGT GGT GAT GCG TAA AGG CGA AGA GCT-3' <BR>
| + | |
| - | BBa_I746908 backbone reverse primer:5'-TAG CGC CTC GTG GTG GTG GTG GTG GTG CAT TAG TAT TTC TCC TCT TTC TCT AGT AGC TAG-3' <BR>
| + | |
| | | | |
| | {{Template:EPFL2013Footer}} | | {{Template:EPFL2013Footer}} |
 "
"