Team:KU Leuven/Project/Glucosemodel/EBF
From 2013.igem.org
| (120 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
<div class="container"> | <div class="container"> | ||
| + | <!--LOGOS--> | ||
| + | |||
| + | <!-- TITLE --> | ||
<div id="header" class="row-fluid"> | <div id="header" class="row-fluid"> | ||
<div class="span12"> | <div class="span12"> | ||
| - | <h3 class="bg-green"> | + | <h3 class="bg-green">The Honeydew System</h3> |
</div> | </div> | ||
</div> | </div> | ||
| + | |||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span6 icon white"> |
| - | < | + | <div class="row-fluid"> |
| - | + | <div class="visible-desktop span3 bg-grey1"> | |
| - | + | <a href="https://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/Design"> | |
| - | </ | + | <i class="livicon activeicon" data-name="pen" data-onparent="true" data-color="white"></i> |
| + | </div> | ||
| + | <div class="span9 icon-text"> | ||
| + | <h3>Designing the Honeydew System</h3> </a> | ||
| + | <p>Want to know more about our constructs?</p> | ||
| + | </div> | ||
| + | </div> | ||
</div> | </div> | ||
| - | |||
| - | + | <div class="span6 icon white"> | |
| - | + | <div class="row-fluid"> | |
| - | + | <div class="farnesene visible-desktop span3 bg-green"> | |
| - | <div | + | <a href="https://2013.igem.org/Team:KU Leuven/Project/Glucosemodel/EBF"> |
| - | + | <i class="livicon activeicon" data-name="bell" data-onparent="true" data-color="white"></i> | |
| - | + | </div> | |
| - | + | <div class="span9 icon-text"> | |
| + | <h3>E-β-Farnesene construction</h3> | ||
| + | </a> | ||
| + | <p>You are here!</p> | ||
| + | </div> | ||
| + | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| - | + | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span6 icon white"> |
| - | < | + | <div class="row-fluid"> |
| - | + | <div class="MeS visible-desktop span3 bg-grey1"> | |
| - | + | <a href="https://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/MeS"> | |
| - | < | + | <i class="livicon activeicon" data-name="lab" data-onparent="true" data-color="white"></i> |
| + | </div> | ||
| + | <div class="span9 icon-text"> | ||
| + | <h3>Methyl Salicylate construction</h3> </a> | ||
| + | <p>BanAphids produce MeS!</p> | ||
| + | </div> | ||
| + | </div> | ||
</div> | </div> | ||
| + | <div class="span6 icon white"> | ||
| + | <div class="row-fluid"> | ||
| + | <div class="parts visible-desktop span3 bg-grey1"> | ||
| + | <a href="https://2013.igem.org/Team:KU_Leuven/Project/Glucosemodel/qPCR"> | ||
| + | <i class="livicon activeicon" data-name="spinner-six" data-onparent="true" data-color="white"></i> | ||
| + | </div> | ||
| + | <div class="span9 icon-text"> | ||
| + | <h3>Methyl salicylate - qPCR</h3> </a> | ||
| + | <p>Wetlab data for the MeS model</p> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
</div> | </div> | ||
| Line 46: | Line 79: | ||
<div id="header" class="row-fluid"> | <div id="header" class="row-fluid"> | ||
<div class="span12"> | <div class="span12"> | ||
| - | + | <h3 class="bg-green">E-β-Farnesene</h3> | |
| - | <h3 class="bg-green"> | + | |
</div> | </div> | ||
</div> | </div> | ||
| Line 54: | Line 86: | ||
<div class="span12 white"> | <div class="span12 white"> | ||
<p align="justify"> | <p align="justify"> | ||
| - | + | In this part, we will give more information about the E-β-farnesene (EBF) part of the project. EBF is an <b>alarm pheromone</b>, released by almost all of the 4000 aphid species known thus far <b>in response to the presence of predators</b> (e.g. the ladybug) or other disturbances. In response to the produced EBF, aphids change their metabolism and turn into a winged form, allowing them to "flee the scene" and thus increase their survival rate. Apart from the short term repelling effect, <b>EBF can also cause long term effects: changes in aphid’s development, fecundity, survival when introduced to different growth stages, etc.</b> These have a negative impact on aphid reproduction. Natural aphid predators such as the ladybugs are also attracted by EBF.</p> | |
| - | + | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
</div> | </div> | ||
| - | < | + | <!--LOGOS--> |
| - | + | ||
| - | + | <br/> | |
| - | + | ||
| - | + | ||
| - | </ | + | |
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span4 icon white"> |
| - | < | + | <div class="row-fluid"> |
| - | + | <div class="visible-desktop span3 bg-farnesene"> | |
| - | + | <a href="#EBF synthase background"> | |
| - | </ | + | <i class="livicon activeicon" data-name="tablet" data-onparent="true" data-color="white"></i> |
| + | </div> | ||
| + | <div class="span7 icon-text"> | ||
| + | <h3>EBF brick background</h3> </a> | ||
| + | <p>Model and genes, pathway and future</p> | ||
| + | </div> | ||
| + | </div> | ||
</div> | </div> | ||
| - | |||
| + | <div class="span4 icon white"> | ||
| + | <div class="row-fluid"> | ||
| + | <div class="MeS visible-desktop span3 bg-green"> | ||
| + | <a href="#Characterisation"> | ||
| + | <i class="livicon activeicon" data-name="trophy" data-onparent="true" data-color="white"></i> | ||
| + | </div> | ||
| + | <div class="span7 icon-text"> | ||
| + | <h3>Brick characterisation</h3> </a> | ||
| + | <p>Aphid experiments, GC-MS and more</p> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| - | <div class="row-fluid"> | + | <div class="span4 icon white"> |
| - | + | <div class="row-fluid"> | |
| - | + | <div class="farnesene visible-desktop span3 bg-background"> | |
| - | + | <a href="#Our bricks"> | |
| - | + | <i class="livicon activeicon" data-name="wrench" data-onparent="true" data-color="white"></i> | |
| - | + | </div> | |
| - | + | <div class="span7 icon-text"> | |
| + | <h3>Our bricks</h3> | ||
| + | </a> | ||
| + | <p>Bricks and wetlab overview</p> | ||
| + | </div> | ||
| + | </div> | ||
</div> | </div> | ||
| + | |||
</div> | </div> | ||
| - | < | + | <!--Tekst--> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <div class="row-fluid"> | + | <div id="header" class="row-fluid"> |
| - | <div class="span12 | + | <div class="span12"> |
| - | < | + | <a id="EBF synthase background"></a> |
| - | < | + | <h3 class="bg-green">General Background of the EBF synthase</h3> |
| - | + | ||
| - | + | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 110: | Line 150: | ||
<div class="span12 white"> | <div class="span12 white"> | ||
<p align="justify"> | <p align="justify"> | ||
| - | + | We cloned and expressed the EBF synthase gene in <i>E. coli</i>. This enzyme will break down (2E,6E)-farnesyl diphosphate into (E)-β-farnesene (EBF) and diphosphate (see reaction scheme below).<br/> | |
| - | </ | + | The enzyme prefers bivalent cations as cofactors; a Mg<sup>2+</sup> concentration of 5 mM should be beneficial for EBF synthase function. The ideal pH for EBF synthase will be between 5.5 and 7.</p> |
| + | <center><img src="https://static.igem.org/mediawiki/2013/5/5e/Reaction.jpg" alt="reaction"/></center> | ||
</div> | </div> | ||
</div> | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<div class="row-fluid"> | <div class="row-fluid"> | ||
<div class="span12 white"> | <div class="span12 white"> | ||
| - | <p align="justify"> | + | <a id="model"></a> |
| - | <b> | + | <h3>The Model and the Genes</h3> |
| + | <p align="justify"> | ||
| + | The EBF construct we designed consists of a <b>constitutive promoter with a <i>lac</i> operator, the EBF synthase itself and a double terminator. We used <a href="http://parts.igem.org/Part:BBa_B0015" target="_blank">BBa_B0015</a> for the double terminator.</b> EBF is not only made by aphids but also by plants and other organisms in a form of biomimicry. We obtained two different sources of the EBF gene. One gene originates from the <b>soil bacterium</b> <i>Streptomyces coelicolor</i> (Centre of Microbial and Plant Genetics of KU Leuven). We chose this plant-residing bacterium because it would be a perfect chassis for the ultimate expression of EBF in our <i>E. coligy</i> system. The other EBF gene is from <b>the plant <i>Artemisia annua</i> (sweet wormwood)</b> and was a kind gift from Professor Peter Brodelius (Kalmar University, Sweden). Here we were inspired with the plant origin. The K<sub>M</sub> for the <i>Artemisia annua</i> protein is calculated at 0.0021 mM, with a Kcat/K<sub>M</sub>=4.5 and a turnover number of 0.0095 s<sup>-1</sup>. For the <i>Streptomyces coelicolor</i> protein the K<sub>M</sub> is 0.0168 mM and the turnover number 0.019 s<sup>-1</sup>. <br/> | ||
| + | |||
| + | Unfortunately, the EBF synthase from <i>Streptomyces coelicolor</i> is a bifunctional enzyme, not only processing β-farnesene but also containing albaflavenone synthase activity. For this reason, we chose to follow up on the <i>Artemisia annua</i> gene and product. | ||
| + | For our construct, our first choice was a medium strength promoter with medium RBS (<a href="http://parts.igem.org/Part:BBa_K608006" target="_blank">BBa_K608006</a>); we nonetheless also made the construct with a strong promoter and RBS. The <i>lac</i> operator in front of the EBF synthase gene will allow us to switch the transcription of the EBF synthase gene on and off.</p> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 131: | Line 171: | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
<div class="span12 white"> | <div class="span12 white"> | ||
| - | + | <a id="pathway"></a> | |
| - | + | <h3>The pathway to E-β-Farnesene</h3> | |
| - | <div class=" | + | <div class="row-fluid"> |
| - | + | <div class="span8"> | |
| - | + | <p align="justify"> | |
| - | + | β-farnesene is a terpenoid that is converted from farnesyl pyrophosphate (FPP) by the enzyme β-farnesene synthase (EC 4.2.3.47). <br/> | |
| - | + | <b>FPP is the precursor of β-farnesene</b>, that is produced by the building blocks, the molecules isopentenyl pyrophosphate (IPP) and its isomer dimethylallylpyrophosphate (DMAPP).<br/> | |
| + | These precursors of farnesyl pyrophosphate can be produced by several metabolic pathways. Most <b>prokaryotes use the non-mevalonate or DXP pathway</b>, producing IPP starting from glyceraldehyde-3-phosphate and pyruvate. <b>Eukaryotes, except for plants, exclusively use the mevalonate pathway</b>, producing IPP starting from acetyl-CoA. Plants use both pathways.</p> | ||
</div> | </div> | ||
| - | <div class=" | + | <div class="span4 greytext"> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <a href="https://static.igem.org/mediawiki/2013/2/2a/Mevalonate_pathway.jpg" target="_blank"> |
| + | <img src="https://static.igem.org/mediawiki/2013/2/2a/Mevalonate_pathway.jpg" alt="Mevalonate Pathway"></a> | ||
| + | <p align="justify">Mevalonate pathway, showing the conversion of acetyl-CoA to general terpenoid precursor IPP and its isomer DMAPP. </p> | ||
</div> | </div> | ||
</div> | </div> | ||
| - | + | <br/><br/> | |
| - | </ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span4"> |
| - | + | <a href="https://static.igem.org/mediawiki/2013/4/48/Non-Mevalonate.jpg" target="_blank"> | |
| - | + | <img src="https://static.igem.org/mediawiki/2013/4/48/Non-Mevalonate.jpg" alt="Non-Mevalonate Pathway"/></a> | |
| - | + | ||
| - | + | ||
</div> | </div> | ||
| - | <div class=" | + | <div class="span8"> |
| - | + | <p align="justify">On the left you can see the non-mevalonate pathway or DXP pathway, showing the conversion of pyruvate and glyceraldehyde-3-phosphate to the terpenoid precursor IPP and its isomer DMAPP.<br/> | |
| + | Pyr = pyruvate, G3P = glyceraldehyde-3-phosphate, DXP = 1-deoxy-D-xylulose 5-phosphate, MEP = 2-C-methylerythritol 4-phosphate, CDP-ME = 4-phosphocytidyl-2-C-methylerythritol, CDP-MEP = 4-phosphocytidyl-2-C-methyl-D-erythritol 2-phosphate, MEcPP = 2-C-methyl-D-erythritol 2,4-cyclopyrophosphate, HMB-PP = (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate, DXS = DXP synthase, DXR = DXP reductase, CMS = CDP-ME synthase, CMK = CDP-ME kinase, MCS = MEcPP synthase, HDS = HMB-PP synthase, HDR = HMB-PP reductase</p> | ||
</div> | </div> | ||
| Line 166: | Line 204: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | |||
| + | <!--Problems--> | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
<div class="span12 white"> | <div class="span12 white"> | ||
| + | <a id="problemssolutions"></a> | ||
| + | <h3>Problems & Solutions Concerning The Pathway</h3> | ||
| + | <p align="justify">FPP is an important precursor, used for the biosynthesis of lots and lots of compounds. Once we insert a plasmid containing the β-farnesene synthase gene, we may obtain only a very small amount of β-farnesene, since the precursor amount wasn't increased and there simply isn’t enough FPP available to produce the amount of β-farnesene to fully use the capacity of the EBF synthase enzyme we brought in.<br/> | ||
| + | A solution may be to co-transform plasmids to engineer a mevalonate pathway in <i>E. coli</i>, thereby upregulating the production of FPP. This larger amount of FPP may then be converted to β-farnesene, creating a large enough amount of this volatile. This was demonstrated many times by J.D. Keasling in <i>S. cerervisiae</i>, while Martin <i>et al.</i>, (2003) implemented this mevalonate pathway in <i>E. coli</i>. In the article, they described their successful efforts to create a high level production of amorphadiene by introducing the mevalonate pathway in <i>E. coli</i>. However, expression of this heterologous pathway led to such an abundance of isoprenoid precursors that cells ceased to grow or mutated to overcome the toxicity. This once again shows the need for a controlled production of the elements in this pathway; too much is equally detrimental as too little.</p> | ||
| + | <br/><br/> | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | + | <div class="span4 greytext"> | |
| - | <div class=" | + | <a href="https://static.igem.org/mediawiki/2013/9/90/Martin_et_al.%2C_2003.png" target="_blank"> |
| - | + | <img src="https://static.igem.org/mediawiki/2013/9/90/Martin_et_al.%2C_2003.png" alt="Martin et al"/></a> | |
| - | + | <p align="justify"><b>The genes involved in the mevalonate pathway</b><br/>(Martin <i>et al.</i>)</p> | |
| - | + | ||
| - | + | ||
</div> | </div> | ||
| - | <div class=" | + | <div class="span8"> |
| - | + | <p align="justify">Since there are <b>eight genes </b>responsible for the mevalonate pathway, Martin <i>et al.</i> decided to split them up into <b>two parts</b>. A first plasmid named <b>pMevT</b>, responsible for the conversion of acetyl-CoA to mevalonate, harboring the <i>atoB</i>, <i>HMGS</i> and <i>tHMGR</i> genes into a pBAD33 vector, and a second one named <b>pMBIS</b>, harboring the <i>ERG12</i>, <i>ERG8</i>, <i>MVD1</i>, <i>idi</i> and <i>ispA</i> genes into a pBBR1MCS-3 plasmid. Coexpression of these two operons in an <i>ispC</i> deficient <i>E. coli</i> strain produced the terpenes, even in the absence of mevalonate, indicating that the mevalonate pathway works. <b>A combined expression of their recombinant mevalonate pathway and the synthetic gene product (ADS in their case) resulted in greatly improved yields</b>.<br/><br/></p> | |
</div> | </div> | ||
| - | + | <p align="justify">Even though we do not need a very high production of EBF it would be definitely better to optimise the pathway, by using the plasmids pMevT and pMBIS, described above. <b>Implementing them into our BanAphids along with the synthetic β-farnesene synthase gene could result into high yields of β-farnesene. This way the amount of EBF can be easily changed via the amount of bacteria used or the concentration of the cofactor Mg<sup>2+</sup></b>.<br/>Due to the short amount of time iGEM offered we did not yet started doing this, but this is definitely something future teams might look into.</p> | |
| + | |||
| + | |||
</div> | </div> | ||
| + | </div> | ||
</div> | </div> | ||
| - | |||
| - | |||
| - | |||
| - | + | <!--Characterisation--> | |
| - | + | <div id="header" class="row-fluid"> | |
| - | + | <div class="span12"> | |
| - | + | <a id="Characterisation"></a> | |
| - | + | <h3 class="bg-green">Characterisation</h3> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 209: | Line 245: | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span12"> |
| - | + | <p align="justify">After a lot of blood, sweat and tears to create the EBF BioBrick we characterised it by means of <a href="#aphid experiments">behavioural aphid experiments</a> as well as investigating the effect of EBF on the amount of aphids and aphid movement. We further characterised this brick with <a href="#GC-MS analysis of EBF">GC-MS analysis</a> and <a href="#SDSPAGE">EBF synthase expression</a>. </p> | |
| - | + | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| - | + | <!--Aphid experiments--> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<div id="header" class="row-fluid"> | <div id="header" class="row-fluid"> | ||
<div class="span12"> | <div class="span12"> | ||
<a id="aphid experiments"></a> | <a id="aphid experiments"></a> | ||
| - | <h3 class="bg- | + | <h3 class="bg-farnesene">Aphid experiments</h3> |
</div> | </div> | ||
</div> | </div> | ||
| Line 239: | Line 268: | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span6"> |
<p align="justify"> | <p align="justify"> | ||
With our EBF synthase constructs ready, we tested them with several aphid experiments.<br/> | With our EBF synthase constructs ready, we tested them with several aphid experiments.<br/> | ||
Our pilot experiment tested the medium strength EBF synthase producing brick <b><a href="http://parts.igem.org/Part:BBa_K1060009" target="_blank">BBa_K1060009</b></a>. We placed aphids on a leaf in the middle of a huge petri dish, an EBF-producing bacterium plate on the left, a control on the right. In <b>the resulting video we observed that the general trend of aphid movement was away from the EBF-producing bacterium. These results suggest our EBF synthase producing bacteria seemed to work.</b> <br/> | Our pilot experiment tested the medium strength EBF synthase producing brick <b><a href="http://parts.igem.org/Part:BBa_K1060009" target="_blank">BBa_K1060009</b></a>. We placed aphids on a leaf in the middle of a huge petri dish, an EBF-producing bacterium plate on the left, a control on the right. In <b>the resulting video we observed that the general trend of aphid movement was away from the EBF-producing bacterium. These results suggest our EBF synthase producing bacteria seemed to work.</b> <br/> | ||
| - | Moreover, we also tried another set-up with our high strength EBF synthase producing brick <b><a href="http://parts.igem.org/Part:BBa_K1060011" target="_blank">BBa_K1060011</b></a>. This time we connected the leaves that were on the EBF-producing bacteria plate with those on the control plate and with the leaf in the middle where the aphids resided. This facilitates movement of the aphids to other leaves. However, there was no significant difference between the amount of aphids on the control leaves versus the <a href="http://parts.igem.org/Part:BBa_K1060011" target="_blank">BBa_K106011</a> leaves. The lac operator in this construct may interfere with the production of EBF.</p> | + | Moreover, we also tried another set-up with our high strength EBF synthase producing brick <b><a href="http://parts.igem.org/Part:BBa_K1060011" target="_blank">BBa_K1060011</b></a>. This time we connected the leaves that were on the EBF-producing bacteria plate with those on the control plate and with the leaf in the middle where the aphids resided. This facilitates movement of the aphids to other leaves. However, there was no significant difference between the amount of aphids on the control leaves versus the <a href="http://parts.igem.org/Part:BBa_K1060011" target="_blank">BBa_K106011</a> leaves. The lac operator in this construct may interfere with the production of significant amounts of EBF.</p> |
</div> | </div> | ||
| - | <div class=" | + | <div class="span6 greytext"> |
| - | <p><iframe width=" | + | <p align="justify"><iframe width="374" height="280" src="//www.youtube.com/embed/n-pMp8-XdUo?rel=0" frameborder="0" allowfullscreen></iframe></p> |
| + | <p align="justify">In this video you will see the first behavioural experiment with aphids and BanAphids. An EBF-producing bacterium plate is placed on the left, a blank on the right, aphids are placed on the leaf in the middle. These first results seem positive, as we can see them moving from left to right. (You may have to watch the video in full screen to clearly see the aphids.)</p> | ||
</div> | </div> | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
| - | + | <p align="justify"> | |
| - | In addition, we also examined the aphid's behavior without a leaf as a starting point. We put 30 aphids in the middle of a huge petri dish, on the left side we placed a leaf with 10µl of EBF-producing bacteria and on the right side we placed a non-treated leaf as control. Thus, we offered the aphids the chance to go searching for food. After 2 hours we counted the number of aphids on the leaves, there were 4 aphids on the leaf where the EBF was produced and 6 aphids on the control leaf, the rest of aphids just walked randomly in the big petri dish. For lack of time, we could unfortunately not repeat this experiment.<br/> | + | In addition, we also examined the aphid's behavior without a leaf as a starting point. We put 30 aphids in the middle of a huge petri dish, on the left side we placed a leaf with 10µl of EBF-producing bacteria and on the right side we placed a non-treated leaf as control. Thus, we offered the aphids the chance to go searching for food. After 2 hours we counted the number of aphids on the leaves, there were 4 aphids on the leaf where the EBF was produced and 6 aphids on the control leaf, the rest of aphids just walked randomly in the big petri dish. For lack of time, we could unfortunately not repeat this experiment.<br/><br/> |
| - | <b>Our pilot experiments | + | <b>Our pilot experiments indicated a trend in the right direction.</b> Several aspects of the setup can still be optimized in the future, for example the amount of bacteria, the strength of the promoter, the ventilation of the setup, the incubation time and the temperature, etc. The reason for this is that the concentration of EBF is essential to trigger the desired response in the aphids. Both too high and too low concentrations will lead to aphid insensitivity. <br/> |
</p> | </p> | ||
</div> | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <div class="row-fluid"> | ||
| + | <div class="span12 white"> | ||
| + | <div class="row-fluid"> | ||
| + | |||
| + | <div class="span12"> | ||
| + | <div class="row-fluid"> | ||
| + | <div class="span5"> | ||
| + | <p align="justify"> | ||
| + | To back-up our pilot experiment, we tested again our medium strength EBF synthase producing brick <b><a href="http://parts.igem.org/Part:BBa_K1060009" target="_blank">BBa_K1060009</b></a>. <br/><br/> | ||
| + | This time we used <b>3 biological repeats</b> for both a control setup (BL21 wild-type) and our EBF producing strain. From an infested <i>Capsicum annuum</i> (‘sweet pepper’ or ‘bell pepper’) plant, leaves of similar sizes were cut and placed in glass containers with sufficient natural ventilation. The aphids were then left for one hour before introducing our bacterial plates. Petri dishes with 10µl of a fresh overnight culture (~OD 1.6) were place under each leaf. The amount of aphids on the top side of each leaf were counted and used as a reference point (t=0). Every half hour the aphids were counted in a standardised manner. The amount of aphids moving on each leaf were also counted at each time point. We observed a trend of more aphids scattering away from the leave in the EBF setup but no statistical differences were seen in the control group (see Figure: Upper panel). <br/> <br/> | ||
| + | <b>When looking at the percentage of aphids moving at each time point, we observed a similar trend of aphids being more agitated in the EBF setup compared to the control (see Figure: Lower panel). This difference was statistically significant (P-value = 0.013) at 180 minutes after introducing our bacteria. </b> | ||
| + | </div> | ||
| + | <div class="span7 greytext"> | ||
| + | <a href="https://static.igem.org/mediawiki/2013/7/7f/Bio_assay_top_aphids.tif" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/7/7f/Bio_assay_top_aphids.tif"></a> | ||
| + | <a href="https://static.igem.org/mediawiki/2013/e/e8/Bio_assay_moving_aphids.tif" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/e/e8/Bio_assay_moving_aphids.tif"></a> <br/> | ||
| + | <p align="justify" style="font-size:12px">BBa_K1060009 (EBF) or BL21 (control) bacterial plates were placed underneath leaves (n=3) from <i>Capsicum annuum</i>. The amount of aphids moving around on the top of each leaf were counted at 0, 30, 60, 90, 150 and 180 minutes after the introduction of the bacteria. The average total count of aphids moving on the 3 leaves for EBF and control are also shown. Upper panel: data are represented as % of aphids compared to time point 0 ± standard error of mean. The average total count of aphids on the 3 leaves for EBF and control are also shown. Lower panel: data are represented as % of aphids moving compared to the amount of aphids on the top side of each leaf ± standard error of mean.</p> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
</div> | </div> | ||
</div> | </div> | ||
| Line 266: | Line 322: | ||
<div id="header" class="row-fluid"> | <div id="header" class="row-fluid"> | ||
<div class="span12"> | <div class="span12"> | ||
| - | <h3 class="bg- | + | <a id="GC-MS analysis of EBF"></a> |
| + | <h3 class="bg-farnesene">GC-MS analysis of EBF</h3> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <div class="row-fluid"> | ||
| + | <div class="span12 white"> | ||
| + | <h4> </h4> | ||
| + | <p align="justify"> | ||
| + | To confirm that our <i>E. coli</i> strain containing our EBF synthase producing brick <b><a href="http://parts.igem.org/Part:BBa_K1060009" target="_blank">BBa_K1060009</b></a> produced EBF, we turned to GC-MS analysis with the help of Dr. Jan Baeten (Centre for Surface Chemistry and Catalysis; Prof. Dirk De Vos). Supernatant of bacterial cultures were extacted 3 times with hexane. GC-MS analyses were carried out using a 7890A Agilent gas chromatograph coupled to a 5977A mass spectrometric detector. The GC was equiped with a HP-5MS capillary column (30m x 0.25 mm x 0.25 µm). 1 µl of each sample was injected using splitless (head pressure 9.15 psi) or pulsed splitless (head pressure 20 psi) injection at a temperature of 250 °C. The initial oven temperature of 40 °C was held for 1 min., ramped at 6 °C / min. to 124 °C, then ramped at 20 °C / min. to 320 °C and finally kept at this temperature for 5 min. The transfer line and ion source were held at 320 °C and 230 °C, respectively. Mass spectra were taken between masses m/z 30-300 with an ionization potential of 70 eV. Retention indices of standards were determined by co-injection of a C7-C30 n-alkane mixture (Supelco) and were compared with published retention indices. | ||
| + | To test if EBF could be detected by this setup, farnesene (Sigma-Aldrich, W383902, mixture of isomers) was used as a standard. In total 16-17 peaks were observed of which the following could be identified with the help of the mass spec and retention index (RI). E-beta-farnesene RI = 1459 (lit. 1458-59), (3Z,6E)-alfa-farnesene RI = 1490 (lit. 1487) and (3E,6E)-alfa-farnesene RI = 1506 (lit. 1506-1510). All samples were measured in duplicate; once in SCAN-modus, splitless injectie of 1 µl, and once in SIM-modus (most sensitive), pulsed splitless injectie of 4 µl. No EBF could be detected for our samples, suggesting that the concentration of EBF produced in our cultures is below the threshold values.</p> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <div id="header" class="row-fluid"> | ||
| + | <div class="span12"> | ||
| + | <a id="SDSPAGE"></a> | ||
| + | <h3 class="bg-farnesene">EBF Synthase Expression Experiment</h3> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 273: | Line 348: | ||
<div class="span12 white"> | <div class="span12 white"> | ||
<div class="span6"> | <div class="span6"> | ||
| + | <h4> </h4> | ||
<p align="justify"> | <p align="justify"> | ||
| - | + | Apart from the GC-MS analysis, we also performed protein expression studies with our EBF Synthase bricks.<br/> | |
| - | Here we show the most interesting results. | + | First, we transformed our 3 different EBF synthase bricks, <a href="http://parts.igem.org/Part:BBa_K1060009" target="_blank">BBa_K1060009</a>, <a href="http://parts.igem.org/Part:BBa_K1060014" target="_blank">BBa_K1060014</a> and <a href="http://parts.igem.org/Part:BBa_K1060011" target="_blank">BBa_K1060011</a>, controlled by a medium-strength promoter; a strong promoter or a combination construct consisting of a Lac operator and medium strength promoter, respectively, into BL21(DE3), an <i>E.coli</i> expression strain. First, we took so-called end-point assays where strains were allowed to grow for 8hrs after inoculation. Bacterial pellets were harvested and proteins extracted.<br/> |
| + | Protein extracts were separated on a 10% SDS-PAGE gel. Since we have no access to a specific antibody we verified the overall protein levels by colloidal coomassie stain comparisons. Here we show the most interesting results.<br/> | ||
| + | <br/> | ||
| + | <b>Figure 1</b> shows some slight additional bands in lane a (around 110kDa and around 60kDa) which is the protein extract from the <i>lacI</i> operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lanes. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band.<br/><br/> | ||
| + | |||
| + | We focussed on BBa_K1060009 in <b>Figure 2 and 3</b> where we show further characterizations: MgCl2 was added to the growth medium as it is suggested to improve solubility and functionality of EBF Synthase. We transformed BBa_K1060009 into DH5a and BL21(DE3), and grew the bacteria with/without MgCl2. Simultaneously we took an "empty" strain as a control, with/without MgCl2. We grew overnight cultures of these 6 strains, inoculated 50ml each to a final Optical Density (600nm) of 0.05 and followed cellular growth over time (Figure 2). Simultaneously, we took samples for protein extraction at the OD600nm's indicated in Figure 5. The growth curves (Figure 4) showed that DH5a, transformed with BBa_K1060009, had growth issues, irrespective of the presence of MgCl2. Interestingly, these cells showed an additional band around 50kDa (Figure 3) which was not observed in the BL21(DE3) transformed strains (data not shown) nor the control strain (Figure 3). Theoretical predictions suggest the EBF Synthase product should run around 66kDa, yet this still needs to be proven.<br/><br/> | ||
| + | |||
| + | <b>Figure 4</b> displays a comparison between the control strain and the BL21(DE3) expression strain, the latter transformed with BBa_K1060009 and in the presence/absence of MgCl2. Here we could not observe the additional band, seen in Figure 5(*).<br/><br/> | ||
| + | |||
| + | Interestingly, the DH5a strains show a reduced growth rate (Figure 2) AND an additional band (Figure 3); a correlation which would be in line with our Flux Balance Analyses results (see Modelling at the cellular level). | ||
| + | |||
| + | Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF Synthase gene product (Figure 1 & 3) and possibly the increased production of a secondary protein (Figure 1). Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which could lead to increased EBF production, equally inhibitory as a too low concentration. | ||
</p> | </p> | ||
</div> | </div> | ||
| - | <div class="span6"> | + | <div class="span6 greytext"> |
<a href="https://static.igem.org/mediawiki/2013/7/76/EBFS_protein.JPG" target="_blank"> | <a href="https://static.igem.org/mediawiki/2013/7/76/EBFS_protein.JPG" target="_blank"> | ||
<img src="https://static.igem.org/mediawiki/2013/7/76/EBFS_protein.JPG"></a> | <img src="https://static.igem.org/mediawiki/2013/7/76/EBFS_protein.JPG"></a> | ||
| + | <p align="justify" style="font-size:12px">Figure 1: Colloidal coommassie stain of endpoint protein extracts from the EBF Synthase biobricks. Differences between the lanes are indicated with a red arrow.</p> | ||
| + | |||
| + | <a href="https://static.igem.org/mediawiki/2013/c/c5/Growthcurves_oct28_EBFS.jpg" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/c/c5/Growthcurves_oct28_EBFS.jpg" alt="growth curves"></a> | ||
| + | <p align="justify" style="font-size:12px">Figure 2: Growth curves of the EBF Synthase expressing bacterial strains. We followed 6 strains in total, as indicated in the figure legend. Strains were grown overnight and a sample of each taken and diluted to an OD600nm of 0.05; our effective starting point. Initially, strains jointly proceeded through lag phase yet in exponential phase, differences in growth rate can be observed.</p> | ||
| + | <a href="https://static.igem.org/mediawiki/2013/6/6e/Protein_oct28_EBFS.jpg" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/6/6e/Protein_oct28_EBFS.jpg" alt="comparative expression"></a> | ||
| + | <p align="justify" style="font-size:12px">Figure 3: Colloidal coomassie stain of protein extracts from a control strain (BL21(DE3)) and an EBF Synthase containing DH5a <i>E. coli</i> strain, each in the presence/absence of 5 mM MgCl2. Samples were taken at increasing optical densities (indicated on the figure), protein extracts separated on a 10% SDS-PAGE and stained with colloidal coomassie. Differences in the banding pattern are indicated with an (*).</p> | ||
| + | <a href="https://static.igem.org/mediawiki/2013/b/b0/Controlproteins_EBFS.jpg" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/b/b0/Controlproteins_EBFS.jpg" alt=control levels"></a> | ||
| + | <p align="justify" style="font-size:12px">Figure 4: Colloidal coomassie stain of protein extracts from a control strain (BL21(DE3)) and an EBF Synthase containing BL21(DE3) <i>E. coli</i> strain, each in the presence/absence of 5 mM MgCl2. Samples were taken at increasing optical densities (indicated on the figure), protein extracts separated on a 10% SDS-PAGE and stained with colloidal coomassie. No differences in the banding pattern could be observed.</p> | ||
| + | |||
</div> | </div> | ||
| Line 286: | Line 385: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | |||
| + | <!--Our Bricks--> | ||
<div id="header" class="row-fluid"> | <div id="header" class="row-fluid"> | ||
<div class="span12"> | <div class="span12"> | ||
| - | <a id=" | + | <a id="bricks"></a><a id="Our bricks"></a> |
| - | <h3 class="bg-green"> | + | <h3 class="bg-green">Our Bricks</h3> |
</div> | </div> | ||
</div> | </div> | ||
| + | |||
| + | <!--Wetlab overview--> | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
<div class="span12 white"> | <div class="span12 white"> | ||
| - | < | + | <a id="wetlab"></a> |
| + | <h3>Gettin' the gene</h3> | ||
| + | <p align="justify"> | ||
| + | In the case of the EBF synthase gene from <i>Streptomyces coelicolor</i>, we amplified this gene with a colony PCR. The EBF synthase gene from <i>Artemisia annua</i> was received in the pET28 vector from professor Brodelius (Kalmar University, Sweden). In this gene an additional <i>EcoRI</i> restriction site was present, which would conflict with the standard iGEM cloning work. Therefore <b>we removed this site via site directed mutagenesis</b> after transferring the gene into the iGEM pSB1C3 backbone. | ||
| + | <br/><br/></p> | ||
| + | |||
| + | <h3>Cutting and pasting</h3> | ||
| + | <p align="justify"> | ||
| + | Once we obtained the target gene (EBF) in the standard pSB1C3 backbone, we started our cloning work. We used plasmid pSB1C3 with a promoter or terminator as chassis, cut this open and inserted the gene of interest. When ligating the insert in front of the double terminator, we cut the vector with <i>EcoRI</i> and <i>XbaI</i>, and the insert with <i>EcoRI</i> and <i>SpeI</i>. The promotor vector on the other hand is cut with <i>SpeI</i> and <i>PstI</i> restriction sites, and the insert is cut with <i>XbaI</i> and <i>PstI</i> restriction sites. This works because <i>SpeI</i> and <i>XbaI</i> are isoschizomers.<br/> | ||
| + | Ligations were performed in parallel in two different ways. In one setup we ligated for 20 minutes at 16 ℃, and in comparison, the second ligation of the same products was conducted at 16 ℃ overnight.<br/> | ||
| + | For transformation, we used both chemically competent cells and electrocompetent cells. Electroporation had a higher efficiency when compared to heat shock transformation. <br/><br/></p> | ||
| + | |||
| + | <h3>Confirmation</h3> | ||
| + | <p align="justify"> | ||
| + | After we observed colonies the next day, we needed to confirm the products. The first step we did was usually a colony PCR to check if the insert was in the vector, this was followed up by digestion confirmation after the plasmid extraction. <b>Only the plasmids which succeeded in both controls were send for sequencing, the final confirmation</b>. | ||
| + | <br/><br/></p> | ||
| - | + | <h3>gBlocks</h3> | |
| - | + | <p align="justify"> | |
| - | + | Meanwhile, we also built the EBF construct with a <i>lac</i> operator between the promoter and gene, using the gBlock principle. We designed the gBlocks, assembled them and ligated the insert into pSB1C3 backbone. The colonies obtained also went through the three confirmation steps mentioned above before we were satisfied. | |
| - | < | + | <br/><br/></p> |
| - | + | ||
| + | <p align="justify"> | ||
| + | For more details on the labwork and the wetlab difficulties as well as how we overcame them, please consult <a href="https://2013.igem.org/Team:KU_Leuven/Journal/EBF/wetlab">our wetlab journal</a>. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <!--Bricks--> | ||
| + | |||
| + | <div class="row-fluid"> | ||
| + | <div class="span12 white"> | ||
| + | <div class="row-fluid"> | ||
| + | <div class="span3"> | ||
| + | <a id="Biobricks"></a> | ||
| + | <h3><br/></h3> | ||
| + | <p align="justify"> | ||
| + | <li><a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1060001" target="_blank">BBa_K1060001</a> This is a coding biobrick with the EBF synthase gene from <i>Streptomyces coelicolor</i> in pSB1C3 backbone and an insert length of 1386bp.</li> | ||
| + | </p> | ||
</div> | </div> | ||
| - | <div class=" | + | <div class="span9"> |
| - | + | <img src="https://static.igem.org/mediawiki/2013/b/b9/BBa_K1060001_gel_pic.jpg"> | |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | |
| - | + | ||
</div> | </div> | ||
| Line 319: | Line 452: | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span3"> |
| - | + | <h3><br/></h3> | |
| - | + | <p align="justify"> | |
| + | <li><a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1060002" target="_blank">BBa_K1060002</a> This is another coding biobrick with EBF synthase gene from <i>Artemisia annua</i> in pSB1C3 backbone and an insert length of 1725bp.</li> | ||
| + | </p> | ||
</div> | </div> | ||
| - | <div class=" | + | <div class="span9"> |
| - | + | <img src="https://static.igem.org/mediawiki/2013/a/af/BBa_K1060002_gel_pic.jpg"> | |
| - | + | ||
</div> | </div> | ||
</div> | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</div> | </div> | ||
</div> | </div> | ||
| Line 343: | Line 469: | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
<div class="span12 white"> | <div class="span12 white"> | ||
| - | + | <div class="row-fluid"> | |
| - | + | ||
| + | <div class="span3"> | ||
| + | <h3><br/></h3> | ||
| + | <p align="justify"> | ||
| + | <li><a href="http://parts.igem.org/Part:BBa_K1060008" target="_blank">BBa_K1060008</a> This is an intermediate biobrick with EBF of <i>Artemisia annua</i> in front of a double terminator. </li> | ||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | <div class="span9"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/5/5a/BBa_K1060008_gel_pic.jpg"> | ||
| + | </div> | ||
| + | |||
| + | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 352: | Line 490: | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
| - | <div class=" | + | <div class="span3"> |
| - | + | <h3><br/></h3> | |
| - | + | <p align="justify"> | |
| - | + | <li><a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1060009" target="_blank">BBa_K1060009</a> This is a generator biobrick with a medium constitutive expression of EBF synthase from <i>Artemisia annua</i> in the pSB1C3 backbone and an insert length of 1924bp.</li> | |
| + | </p> | ||
</div> | </div> | ||
| - | <div class=" | + | <div class="span9"> |
| - | < | + | <img src="https://static.igem.org/mediawiki/2013/a/a1/BBa_K1060009_gel_pic.jpg"> |
</div> | </div> | ||
| Line 368: | Line 507: | ||
<div class="row-fluid"> | <div class="row-fluid"> | ||
<div class="span12 white"> | <div class="span12 white"> | ||
| - | + | <div class="row-fluid"> | |
| + | |||
| + | <div class="span3"> | ||
| + | <h3><br/></h3> | ||
| + | <p align="justify"> | ||
| + | <li><a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1060011" target="_blank">BBa_K1060011</a> This is a generator biobrick with a medium constitutive expression of EBF synthase from <i>Artemisia annua</i> AND a <i>lac</i> operator after the promoter and an insert length of 1965bp.</li> | ||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | <div class="span9"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/b/b1/BBa_K10600011_gel_pic.jpg"> | ||
| + | </div> | ||
| + | |||
| + | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| + | </ol> | ||
| + | |||
| + | <!--References--> | ||
<div id="header" class="row-fluid"> | <div id="header" class="row-fluid"> | ||
Latest revision as of 03:06, 29 October 2013
Secret garden
Congratulations! You've found our secret garden! Follow the instructions below and win a great prize at the World jamboree!
- A video shows that two of our team members are having great fun at our favourite company. Do you know the name of the second member that appears in the video?
- For one of our models we had to do very extensive computations. To prevent our own computers from overheating and to keep the temperature in our iGEM room at a normal level, we used a supercomputer. Which centre maintains this supercomputer? (Dutch abbreviation)
- We organised a symposium with a debate, some seminars and 2 iGEM project presentations. An iGEM team came all the way from the Netherlands to present their project. What is the name of their city?
Now put all of these in this URL:https://2013.igem.org/Team:KU_Leuven/(firstname)(abbreviation)(city), (loose the brackets and put everything in lowercase) and follow the very last instruction to get your special jamboree prize!


The Honeydew System
E-β-Farnesene
In this part, we will give more information about the E-β-farnesene (EBF) part of the project. EBF is an alarm pheromone, released by almost all of the 4000 aphid species known thus far in response to the presence of predators (e.g. the ladybug) or other disturbances. In response to the produced EBF, aphids change their metabolism and turn into a winged form, allowing them to "flee the scene" and thus increase their survival rate. Apart from the short term repelling effect, EBF can also cause long term effects: changes in aphid’s development, fecundity, survival when introduced to different growth stages, etc. These have a negative impact on aphid reproduction. Natural aphid predators such as the ladybugs are also attracted by EBF.
We cloned and expressed the EBF synthase gene in E. coli. This enzyme will break down (2E,6E)-farnesyl diphosphate into (E)-β-farnesene (EBF) and diphosphate (see reaction scheme below).
The enzyme prefers bivalent cations as cofactors; a Mg2+ concentration of 5 mM should be beneficial for EBF synthase function. The ideal pH for EBF synthase will be between 5.5 and 7.

The Model and the Genes
The EBF construct we designed consists of a constitutive promoter with a lac operator, the EBF synthase itself and a double terminator. We used BBa_B0015 for the double terminator. EBF is not only made by aphids but also by plants and other organisms in a form of biomimicry. We obtained two different sources of the EBF gene. One gene originates from the soil bacterium Streptomyces coelicolor (Centre of Microbial and Plant Genetics of KU Leuven). We chose this plant-residing bacterium because it would be a perfect chassis for the ultimate expression of EBF in our E. coligy system. The other EBF gene is from the plant Artemisia annua (sweet wormwood) and was a kind gift from Professor Peter Brodelius (Kalmar University, Sweden). Here we were inspired with the plant origin. The KM for the Artemisia annua protein is calculated at 0.0021 mM, with a Kcat/KM=4.5 and a turnover number of 0.0095 s-1. For the Streptomyces coelicolor protein the KM is 0.0168 mM and the turnover number 0.019 s-1.
Unfortunately, the EBF synthase from Streptomyces coelicolor is a bifunctional enzyme, not only processing β-farnesene but also containing albaflavenone synthase activity. For this reason, we chose to follow up on the Artemisia annua gene and product.
For our construct, our first choice was a medium strength promoter with medium RBS (BBa_K608006); we nonetheless also made the construct with a strong promoter and RBS. The lac operator in front of the EBF synthase gene will allow us to switch the transcription of the EBF synthase gene on and off.
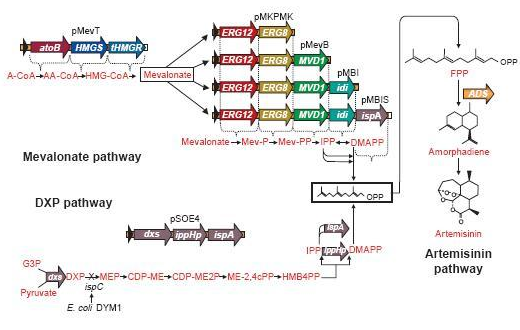
The pathway to E-β-Farnesene
β-farnesene is a terpenoid that is converted from farnesyl pyrophosphate (FPP) by the enzyme β-farnesene synthase (EC 4.2.3.47).
FPP is the precursor of β-farnesene, that is produced by the building blocks, the molecules isopentenyl pyrophosphate (IPP) and its isomer dimethylallylpyrophosphate (DMAPP).
These precursors of farnesyl pyrophosphate can be produced by several metabolic pathways. Most prokaryotes use the non-mevalonate or DXP pathway, producing IPP starting from glyceraldehyde-3-phosphate and pyruvate. Eukaryotes, except for plants, exclusively use the mevalonate pathway, producing IPP starting from acetyl-CoA. Plants use both pathways.
On the left you can see the non-mevalonate pathway or DXP pathway, showing the conversion of pyruvate and glyceraldehyde-3-phosphate to the terpenoid precursor IPP and its isomer DMAPP.
Pyr = pyruvate, G3P = glyceraldehyde-3-phosphate, DXP = 1-deoxy-D-xylulose 5-phosphate, MEP = 2-C-methylerythritol 4-phosphate, CDP-ME = 4-phosphocytidyl-2-C-methylerythritol, CDP-MEP = 4-phosphocytidyl-2-C-methyl-D-erythritol 2-phosphate, MEcPP = 2-C-methyl-D-erythritol 2,4-cyclopyrophosphate, HMB-PP = (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate, DXS = DXP synthase, DXR = DXP reductase, CMS = CDP-ME synthase, CMK = CDP-ME kinase, MCS = MEcPP synthase, HDS = HMB-PP synthase, HDR = HMB-PP reductase
Problems & Solutions Concerning The Pathway
FPP is an important precursor, used for the biosynthesis of lots and lots of compounds. Once we insert a plasmid containing the β-farnesene synthase gene, we may obtain only a very small amount of β-farnesene, since the precursor amount wasn't increased and there simply isn’t enough FPP available to produce the amount of β-farnesene to fully use the capacity of the EBF synthase enzyme we brought in.
A solution may be to co-transform plasmids to engineer a mevalonate pathway in E. coli, thereby upregulating the production of FPP. This larger amount of FPP may then be converted to β-farnesene, creating a large enough amount of this volatile. This was demonstrated many times by J.D. Keasling in S. cerervisiae, while Martin et al., (2003) implemented this mevalonate pathway in E. coli. In the article, they described their successful efforts to create a high level production of amorphadiene by introducing the mevalonate pathway in E. coli. However, expression of this heterologous pathway led to such an abundance of isoprenoid precursors that cells ceased to grow or mutated to overcome the toxicity. This once again shows the need for a controlled production of the elements in this pathway; too much is equally detrimental as too little.
Since there are eight genes responsible for the mevalonate pathway, Martin et al. decided to split them up into two parts. A first plasmid named pMevT, responsible for the conversion of acetyl-CoA to mevalonate, harboring the atoB, HMGS and tHMGR genes into a pBAD33 vector, and a second one named pMBIS, harboring the ERG12, ERG8, MVD1, idi and ispA genes into a pBBR1MCS-3 plasmid. Coexpression of these two operons in an ispC deficient E. coli strain produced the terpenes, even in the absence of mevalonate, indicating that the mevalonate pathway works. A combined expression of their recombinant mevalonate pathway and the synthetic gene product (ADS in their case) resulted in greatly improved yields.
Even though we do not need a very high production of EBF it would be definitely better to optimise the pathway, by using the plasmids pMevT and pMBIS, described above. Implementing them into our BanAphids along with the synthetic β-farnesene synthase gene could result into high yields of β-farnesene. This way the amount of EBF can be easily changed via the amount of bacteria used or the concentration of the cofactor Mg2+.
Due to the short amount of time iGEM offered we did not yet started doing this, but this is definitely something future teams might look into.
After a lot of blood, sweat and tears to create the EBF BioBrick we characterised it by means of behavioural aphid experiments as well as investigating the effect of EBF on the amount of aphids and aphid movement. We further characterised this brick with GC-MS analysis and EBF synthase expression.
With our EBF synthase constructs ready, we tested them with several aphid experiments.
Our pilot experiment tested the medium strength EBF synthase producing brick BBa_K1060009. We placed aphids on a leaf in the middle of a huge petri dish, an EBF-producing bacterium plate on the left, a control on the right. In the resulting video we observed that the general trend of aphid movement was away from the EBF-producing bacterium. These results suggest our EBF synthase producing bacteria seemed to work.
Moreover, we also tried another set-up with our high strength EBF synthase producing brick BBa_K1060011. This time we connected the leaves that were on the EBF-producing bacteria plate with those on the control plate and with the leaf in the middle where the aphids resided. This facilitates movement of the aphids to other leaves. However, there was no significant difference between the amount of aphids on the control leaves versus the BBa_K106011 leaves. The lac operator in this construct may interfere with the production of significant amounts of EBF.
In this video you will see the first behavioural experiment with aphids and BanAphids. An EBF-producing bacterium plate is placed on the left, a blank on the right, aphids are placed on the leaf in the middle. These first results seem positive, as we can see them moving from left to right. (You may have to watch the video in full screen to clearly see the aphids.)
In addition, we also examined the aphid's behavior without a leaf as a starting point. We put 30 aphids in the middle of a huge petri dish, on the left side we placed a leaf with 10µl of EBF-producing bacteria and on the right side we placed a non-treated leaf as control. Thus, we offered the aphids the chance to go searching for food. After 2 hours we counted the number of aphids on the leaves, there were 4 aphids on the leaf where the EBF was produced and 6 aphids on the control leaf, the rest of aphids just walked randomly in the big petri dish. For lack of time, we could unfortunately not repeat this experiment.
Our pilot experiments indicated a trend in the right direction. Several aspects of the setup can still be optimized in the future, for example the amount of bacteria, the strength of the promoter, the ventilation of the setup, the incubation time and the temperature, etc. The reason for this is that the concentration of EBF is essential to trigger the desired response in the aphids. Both too high and too low concentrations will lead to aphid insensitivity.
To back-up our pilot experiment, we tested again our medium strength EBF synthase producing brick BBa_K1060009.
This time we used 3 biological repeats for both a control setup (BL21 wild-type) and our EBF producing strain. From an infested Capsicum annuum (‘sweet pepper’ or ‘bell pepper’) plant, leaves of similar sizes were cut and placed in glass containers with sufficient natural ventilation. The aphids were then left for one hour before introducing our bacterial plates. Petri dishes with 10µl of a fresh overnight culture (~OD 1.6) were place under each leaf. The amount of aphids on the top side of each leaf were counted and used as a reference point (t=0). Every half hour the aphids were counted in a standardised manner. The amount of aphids moving on each leaf were also counted at each time point. We observed a trend of more aphids scattering away from the leave in the EBF setup but no statistical differences were seen in the control group (see Figure: Upper panel).
When looking at the percentage of aphids moving at each time point, we observed a similar trend of aphids being more agitated in the EBF setup compared to the control (see Figure: Lower panel). This difference was statistically significant (P-value = 0.013) at 180 minutes after introducing our bacteria.
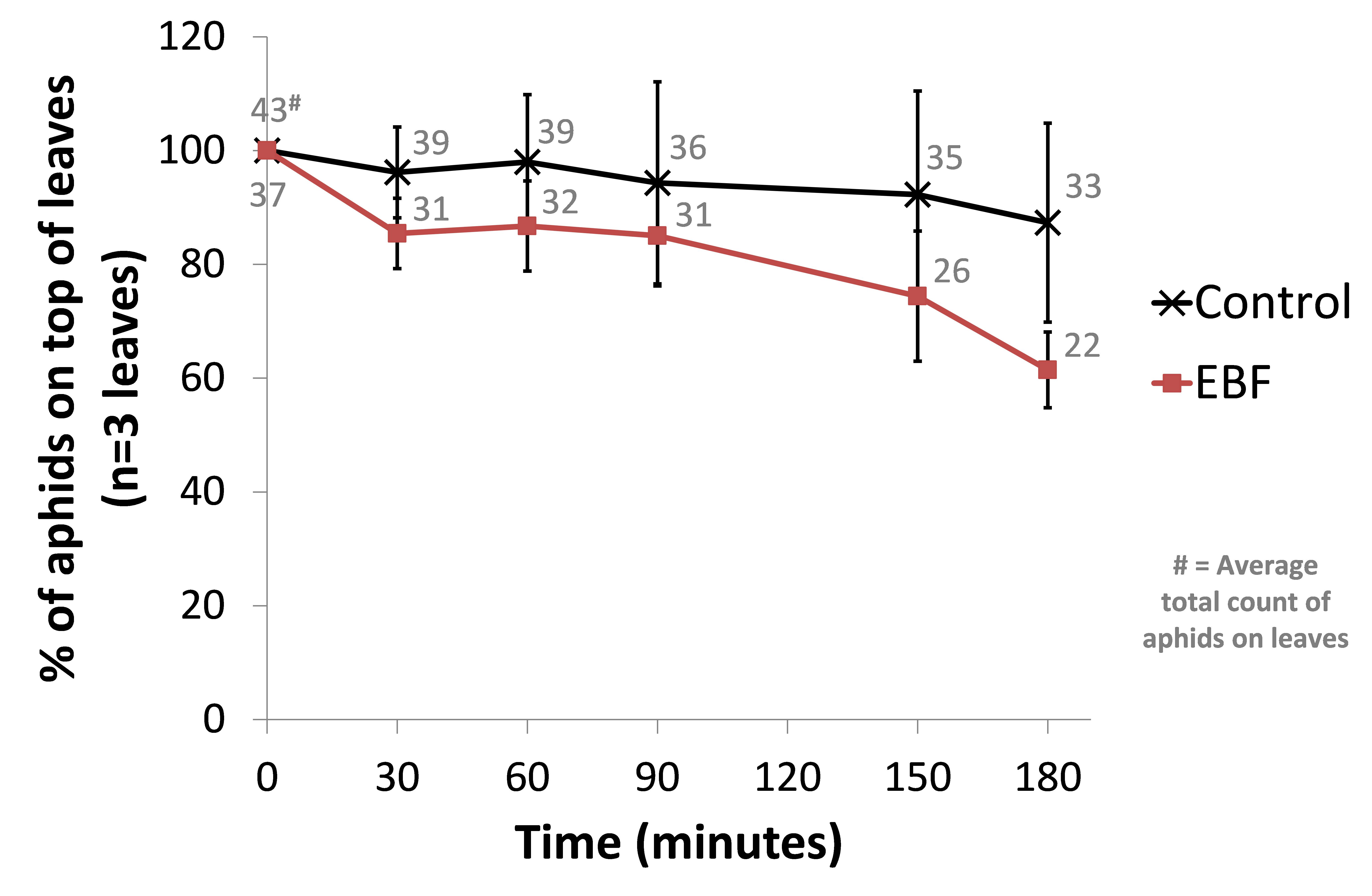

BBa_K1060009 (EBF) or BL21 (control) bacterial plates were placed underneath leaves (n=3) from Capsicum annuum. The amount of aphids moving around on the top of each leaf were counted at 0, 30, 60, 90, 150 and 180 minutes after the introduction of the bacteria. The average total count of aphids moving on the 3 leaves for EBF and control are also shown. Upper panel: data are represented as % of aphids compared to time point 0 ± standard error of mean. The average total count of aphids on the 3 leaves for EBF and control are also shown. Lower panel: data are represented as % of aphids moving compared to the amount of aphids on the top side of each leaf ± standard error of mean.
To confirm that our E. coli strain containing our EBF synthase producing brick BBa_K1060009 produced EBF, we turned to GC-MS analysis with the help of Dr. Jan Baeten (Centre for Surface Chemistry and Catalysis; Prof. Dirk De Vos). Supernatant of bacterial cultures were extacted 3 times with hexane. GC-MS analyses were carried out using a 7890A Agilent gas chromatograph coupled to a 5977A mass spectrometric detector. The GC was equiped with a HP-5MS capillary column (30m x 0.25 mm x 0.25 µm). 1 µl of each sample was injected using splitless (head pressure 9.15 psi) or pulsed splitless (head pressure 20 psi) injection at a temperature of 250 °C. The initial oven temperature of 40 °C was held for 1 min., ramped at 6 °C / min. to 124 °C, then ramped at 20 °C / min. to 320 °C and finally kept at this temperature for 5 min. The transfer line and ion source were held at 320 °C and 230 °C, respectively. Mass spectra were taken between masses m/z 30-300 with an ionization potential of 70 eV. Retention indices of standards were determined by co-injection of a C7-C30 n-alkane mixture (Supelco) and were compared with published retention indices. To test if EBF could be detected by this setup, farnesene (Sigma-Aldrich, W383902, mixture of isomers) was used as a standard. In total 16-17 peaks were observed of which the following could be identified with the help of the mass spec and retention index (RI). E-beta-farnesene RI = 1459 (lit. 1458-59), (3Z,6E)-alfa-farnesene RI = 1490 (lit. 1487) and (3E,6E)-alfa-farnesene RI = 1506 (lit. 1506-1510). All samples were measured in duplicate; once in SCAN-modus, splitless injectie of 1 µl, and once in SIM-modus (most sensitive), pulsed splitless injectie of 4 µl. No EBF could be detected for our samples, suggesting that the concentration of EBF produced in our cultures is below the threshold values.
Apart from the GC-MS analysis, we also performed protein expression studies with our EBF Synthase bricks.
First, we transformed our 3 different EBF synthase bricks, BBa_K1060009, BBa_K1060014 and BBa_K1060011, controlled by a medium-strength promoter; a strong promoter or a combination construct consisting of a Lac operator and medium strength promoter, respectively, into BL21(DE3), an E.coli expression strain. First, we took so-called end-point assays where strains were allowed to grow for 8hrs after inoculation. Bacterial pellets were harvested and proteins extracted.
Protein extracts were separated on a 10% SDS-PAGE gel. Since we have no access to a specific antibody we verified the overall protein levels by colloidal coomassie stain comparisons. Here we show the most interesting results.
Figure 1 shows some slight additional bands in lane a (around 110kDa and around 60kDa) which is the protein extract from the lacI operator medium strength promoter construct. These bands are less clear in the medium and high strength promoter lanes. The expected size of the EBF synthase protein is around 66kDa which could fit with the lower band.
We focussed on BBa_K1060009 in Figure 2 and 3 where we show further characterizations: MgCl2 was added to the growth medium as it is suggested to improve solubility and functionality of EBF Synthase. We transformed BBa_K1060009 into DH5a and BL21(DE3), and grew the bacteria with/without MgCl2. Simultaneously we took an "empty" strain as a control, with/without MgCl2. We grew overnight cultures of these 6 strains, inoculated 50ml each to a final Optical Density (600nm) of 0.05 and followed cellular growth over time (Figure 2). Simultaneously, we took samples for protein extraction at the OD600nm's indicated in Figure 5. The growth curves (Figure 4) showed that DH5a, transformed with BBa_K1060009, had growth issues, irrespective of the presence of MgCl2. Interestingly, these cells showed an additional band around 50kDa (Figure 3) which was not observed in the BL21(DE3) transformed strains (data not shown) nor the control strain (Figure 3). Theoretical predictions suggest the EBF Synthase product should run around 66kDa, yet this still needs to be proven.
Figure 4 displays a comparison between the control strain and the BL21(DE3) expression strain, the latter transformed with BBa_K1060009 and in the presence/absence of MgCl2. Here we could not observe the additional band, seen in Figure 5(*).
Interestingly, the DH5a strains show a reduced growth rate (Figure 2) AND an additional band (Figure 3); a correlation which would be in line with our Flux Balance Analyses results (see Modelling at the cellular level).
Gel extraction and Mass Spectrometry based identification will confirm if these bands represent the EBF Synthase gene product (Figure 1 & 3) and possibly the increased production of a secondary protein (Figure 1). Interestingly, the lacI medium promoter construct did not influence aphid behaviour. Possibly the expression of EBF synthase is just too high, which could lead to increased EBF production, equally inhibitory as a too low concentration.

Figure 1: Colloidal coommassie stain of endpoint protein extracts from the EBF Synthase biobricks. Differences between the lanes are indicated with a red arrow.
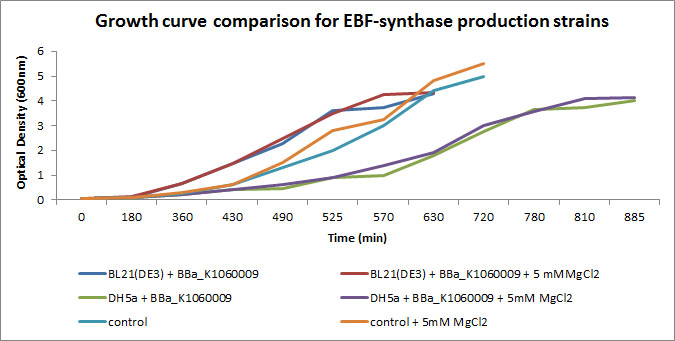
Figure 2: Growth curves of the EBF Synthase expressing bacterial strains. We followed 6 strains in total, as indicated in the figure legend. Strains were grown overnight and a sample of each taken and diluted to an OD600nm of 0.05; our effective starting point. Initially, strains jointly proceeded through lag phase yet in exponential phase, differences in growth rate can be observed.

Figure 3: Colloidal coomassie stain of protein extracts from a control strain (BL21(DE3)) and an EBF Synthase containing DH5a E. coli strain, each in the presence/absence of 5 mM MgCl2. Samples were taken at increasing optical densities (indicated on the figure), protein extracts separated on a 10% SDS-PAGE and stained with colloidal coomassie. Differences in the banding pattern are indicated with an (*).

Figure 4: Colloidal coomassie stain of protein extracts from a control strain (BL21(DE3)) and an EBF Synthase containing BL21(DE3) E. coli strain, each in the presence/absence of 5 mM MgCl2. Samples were taken at increasing optical densities (indicated on the figure), protein extracts separated on a 10% SDS-PAGE and stained with colloidal coomassie. No differences in the banding pattern could be observed.
Gettin' the gene
In the case of the EBF synthase gene from Streptomyces coelicolor, we amplified this gene with a colony PCR. The EBF synthase gene from Artemisia annua was received in the pET28 vector from professor Brodelius (Kalmar University, Sweden). In this gene an additional EcoRI restriction site was present, which would conflict with the standard iGEM cloning work. Therefore we removed this site via site directed mutagenesis after transferring the gene into the iGEM pSB1C3 backbone.
Cutting and pasting
Once we obtained the target gene (EBF) in the standard pSB1C3 backbone, we started our cloning work. We used plasmid pSB1C3 with a promoter or terminator as chassis, cut this open and inserted the gene of interest. When ligating the insert in front of the double terminator, we cut the vector with EcoRI and XbaI, and the insert with EcoRI and SpeI. The promotor vector on the other hand is cut with SpeI and PstI restriction sites, and the insert is cut with XbaI and PstI restriction sites. This works because SpeI and XbaI are isoschizomers.
Ligations were performed in parallel in two different ways. In one setup we ligated for 20 minutes at 16 ℃, and in comparison, the second ligation of the same products was conducted at 16 ℃ overnight.
For transformation, we used both chemically competent cells and electrocompetent cells. Electroporation had a higher efficiency when compared to heat shock transformation.
Confirmation
After we observed colonies the next day, we needed to confirm the products. The first step we did was usually a colony PCR to check if the insert was in the vector, this was followed up by digestion confirmation after the plasmid extraction. Only the plasmids which succeeded in both controls were send for sequencing, the final confirmation.
gBlocks
Meanwhile, we also built the EBF construct with a lac operator between the promoter and gene, using the gBlock principle. We designed the gBlocks, assembled them and ligated the insert into pSB1C3 backbone. The colonies obtained also went through the three confirmation steps mentioned above before we were satisfied.
For more details on the labwork and the wetlab difficulties as well as how we overcame them, please consult our wetlab journal.





Kajiwara S., Fraser P., Kondo K., Misawa N., Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid synthesis in Escherichia coli, Biochem J. 324, 421-426 (1997).
Martin V., Pitera D., Withers S., Newman J., Keasling J., Engineering a mevalonate pathway in Escherichia coli for production of terpenoids, Nature Biotechnology 21(7), 796-802 (2003).
 "
"