Team:DTU-Denmark/Notebook/26 July 2013
From 2013.igem.org
(→Conclusions and Discussion) |
|||
| (17 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:DTU-Denmark/Templates/StartPage|26 July 2013}} | {{:Team:DTU-Denmark/Templates/StartPage|26 July 2013}} | ||
| - | + | Navigate to the [[Team:DTU-Denmark/Notebook/25_July_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/27_July_2013|Next]] Entry | |
| - | =115= | + | =Lab 115= |
<hr/> | <hr/> | ||
==Main purpose== | ==Main purpose== | ||
| - | |||
| + | Investigate nitrite stability in anaerobic, untransformed ''E. coli'' | ||
==Who was in the lab== | ==Who was in the lab== | ||
| Line 12: | Line 12: | ||
==Procedure== | ==Procedure== | ||
| + | <hr> | ||
| + | |||
| + | Following [[Team:DTU-Denmark/Methods/Determining_concentration_of_nitrogen_compounds/Experiment_1a| Experiment 1a]] in order to ensure that any produced nitrite from Mutant 1 will not be consumed by any of the pathways in native ''E. coli''. | ||
| + | |||
| + | ==Results== | ||
| + | <hr> | ||
| + | |||
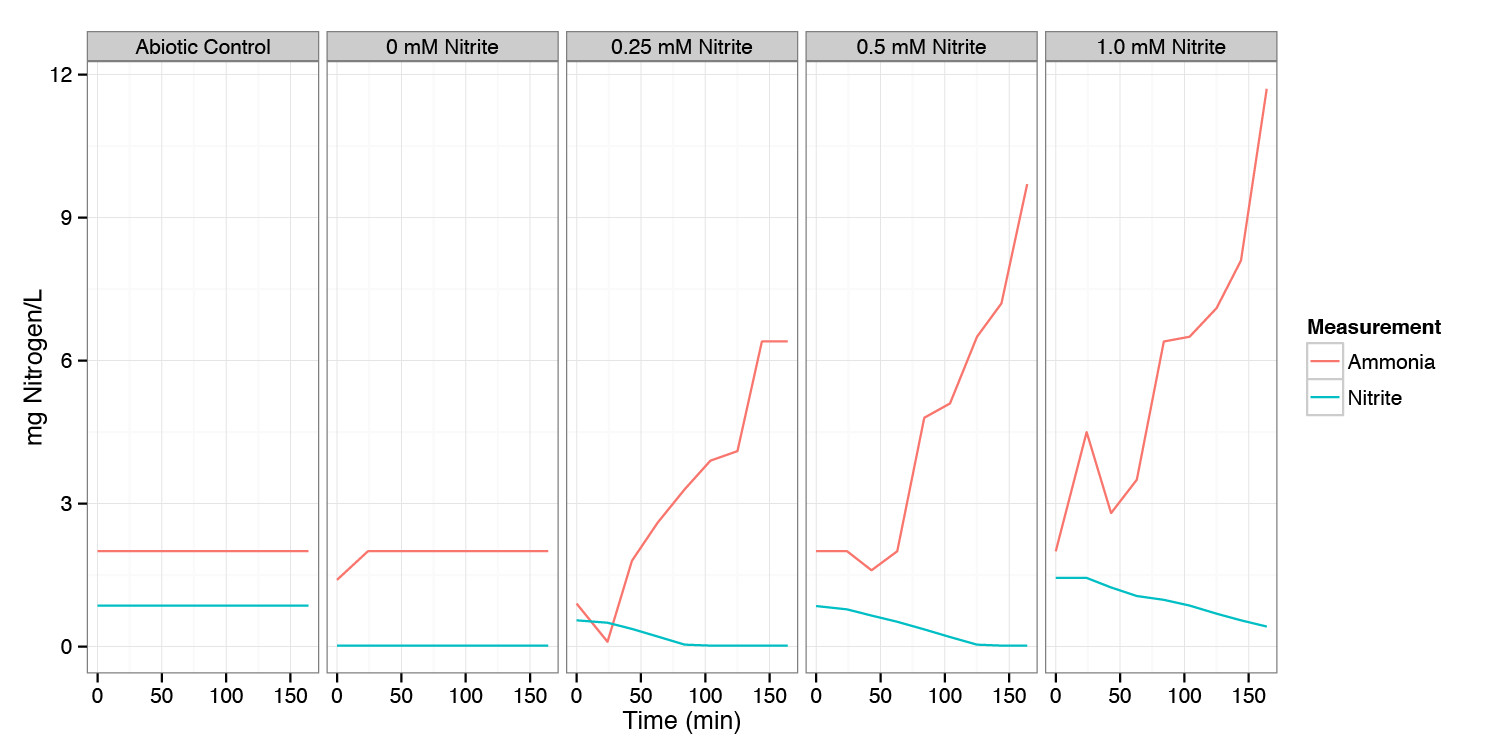
| + | [[File:DTU-Experiment-1a-results.png|600px]] | ||
| + | |||
| + | Mg of Nitrogen (as Nitrite or Ammonia) per liter measured for varying concentrations of Nitrite added at t=0. | ||
| + | All the experiments were performed anaerobically. | ||
| + | Samples were measured every 20 min for 3 hours. | ||
| + | |||
| + | |||
| + | == Conclusions and Discussion == | ||
| + | <hr> | ||
| + | |||
| + | Under anaerobic conditions, nitrite is consumed and converted to ammonium. Since we want to convert the nitrite to nitrous oxide, a functional prototype will require either that the pathway to nitrous oxide be designed to outcompete this pathway, or that this pathway that consumes nitrite be knocked out (and the transformants grown in a medium containing plentiful ammonium so they do not need to synthesize it). | ||
| + | |||
| + | =Lab 208= | ||
<hr/> | <hr/> | ||
| + | ==Main purpose== | ||
| + | <hr/> | ||
| + | * miniprep of TAT, Sec, and Nir for sequencing | ||
| + | * LB medium preparation (2L) | ||
| + | *Pick up new PAO1 cultures | ||
| + | *Make new colony PCR to get Nir from the newly acquired colonies. | ||
| + | |||
| + | ==Who was in the lab== | ||
| + | <hr/> | ||
| + | Kristian, Julia | ||
| + | |||
| + | ==Procedure== | ||
| + | <hr/> | ||
| + | PCRs: | ||
| + | *pZA21::RFP with primer pair 13 58C and 3:00. | ||
| + | *araBAD from K808000 with primer pair 12 61C and 1:00 | ||
| + | *Colony PCR with first PAO1 colony but with a gradient of concentrations from 1x to 500x | ||
| + | *Colony PCR with the 4 new plates of PAO1, concentration x1 | ||
==Results== | ==Results== | ||
<hr/> | <hr/> | ||
| + | See tomorrows gel. | ||
==Conclusion== | ==Conclusion== | ||
<hr/> | <hr/> | ||
| + | Nothing to conclude from today's work. | ||
| + | |||
Navigate to the [[Team:DTU-Denmark/Notebook/25_July_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/27_July_2013|Next]] Entry | Navigate to the [[Team:DTU-Denmark/Notebook/25_July_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/27_July_2013|Next]] Entry | ||
{{:Team:DTU-Denmark/Templates/EndPage}} | {{:Team:DTU-Denmark/Templates/EndPage}} | ||
Latest revision as of 13:59, 1 October 2013
26 July 2013
Contents |
Lab 115
Main purpose
Investigate nitrite stability in anaerobic, untransformed E. coli
Who was in the lab
Helen, Kasia
Procedure
Following Experiment 1a in order to ensure that any produced nitrite from Mutant 1 will not be consumed by any of the pathways in native E. coli.
Results
Mg of Nitrogen (as Nitrite or Ammonia) per liter measured for varying concentrations of Nitrite added at t=0. All the experiments were performed anaerobically. Samples were measured every 20 min for 3 hours.
Conclusions and Discussion
Under anaerobic conditions, nitrite is consumed and converted to ammonium. Since we want to convert the nitrite to nitrous oxide, a functional prototype will require either that the pathway to nitrous oxide be designed to outcompete this pathway, or that this pathway that consumes nitrite be knocked out (and the transformants grown in a medium containing plentiful ammonium so they do not need to synthesize it).
Lab 208
Main purpose
- miniprep of TAT, Sec, and Nir for sequencing
- LB medium preparation (2L)
- Pick up new PAO1 cultures
- Make new colony PCR to get Nir from the newly acquired colonies.
Who was in the lab
Kristian, Julia
Procedure
PCRs:
- pZA21::RFP with primer pair 13 58C and 3:00.
- araBAD from K808000 with primer pair 12 61C and 1:00
- Colony PCR with first PAO1 colony but with a gradient of concentrations from 1x to 500x
- Colony PCR with the 4 new plates of PAO1, concentration x1
Results
See tomorrows gel.
Conclusion
Nothing to conclude from today's work.
Navigate to the Previous or the Next Entry
 "
"