Team:DTU-Denmark/Notebook/8 July 2013
From 2013.igem.org
(→208) |
|||
| (5 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{:Team:DTU-Denmark/Templates/StartPage|}} | + | {{:Team:DTU-Denmark/Templates/StartPage|8 July 2013}} |
| - | + | Navigate to the [[Team:DTU-Denmark/Notebook/7_July_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/9_July_2013|Next]] Entry | |
| - | =208= | + | =Lab 208= |
<hr/> | <hr/> | ||
==Main purpose== | ==Main purpose== | ||
<hr/> | <hr/> | ||
| - | Run gel with 1 uL AMO, 10 uL AMO, 20 uL AMO genes from | + | Run gel with 1 uL AMO, 10 uL AMO, 20 uL AMO genes from ''Nitrosomonas europaea''. |
Purification and extraction of the gel for 10 uL AMO. | Purification and extraction of the gel for 10 uL AMO. | ||
Run 2nd gel with all the sample of 10 uL AMO. | Run 2nd gel with all the sample of 10 uL AMO. | ||
| - | Run gel with HAO and CYC genes | + | Run gel with HAO and CYC genes ''Nitrosomonas europaea''. |
==Who was in the lab== | ==Who was in the lab== | ||
| Line 61: | Line 61: | ||
[[File:2013-07-08_AMO colony PCR.jpg | 600px]] | [[File:2013-07-08_AMO colony PCR.jpg | 600px]] | ||
| + | ==Conclusions== | ||
| + | <hr/> | ||
===Conclusion for AMO=== | ===Conclusion for AMO=== | ||
The bands for 10 AMO are thick and it is not visible how many base-pairs they have, so gel extraction and purification is needed. | The bands for 10 AMO are thick and it is not visible how many base-pairs they have, so gel extraction and purification is needed. | ||
Latest revision as of 20:39, 16 September 2013
8 July 2013
Contents |
Lab 208
Main purpose
Run gel with 1 uL AMO, 10 uL AMO, 20 uL AMO genes from Nitrosomonas europaea. Purification and extraction of the gel for 10 uL AMO. Run 2nd gel with all the sample of 10 uL AMO. Run gel with HAO and CYC genes Nitrosomonas europaea.
Who was in the lab
Kristian, Ariadni, Gosia
Procedure
Run 1st gel (AMO):
- 1ul of dye
- 5ul of sample
- 5ul Kb ladder
Purification of first gel for 10 AMO according to the protocol QIA gel extraction protocol. (given in the Kit)
Run 2nd gel (AMO):
- 9ul of dye
- around 20 ul of each sample
- 12 ul of ladder
Purification of second gel according to the same protocol.
Measure the concentration of the purified AMO using Nanodrop (pouring together both samples).
Run 3rd gel (HAO,CYC):
- 1ul of dye
- 5ul of sample
- 5ul Kb ladder
Purification of DNA of both HAO and CYC.
Results
Nanodrop measurement:
- 30.4 ng/ul (AMO)
- 14.4 ng/ul (HAO)
- 11.7 ng/ul (CYC)
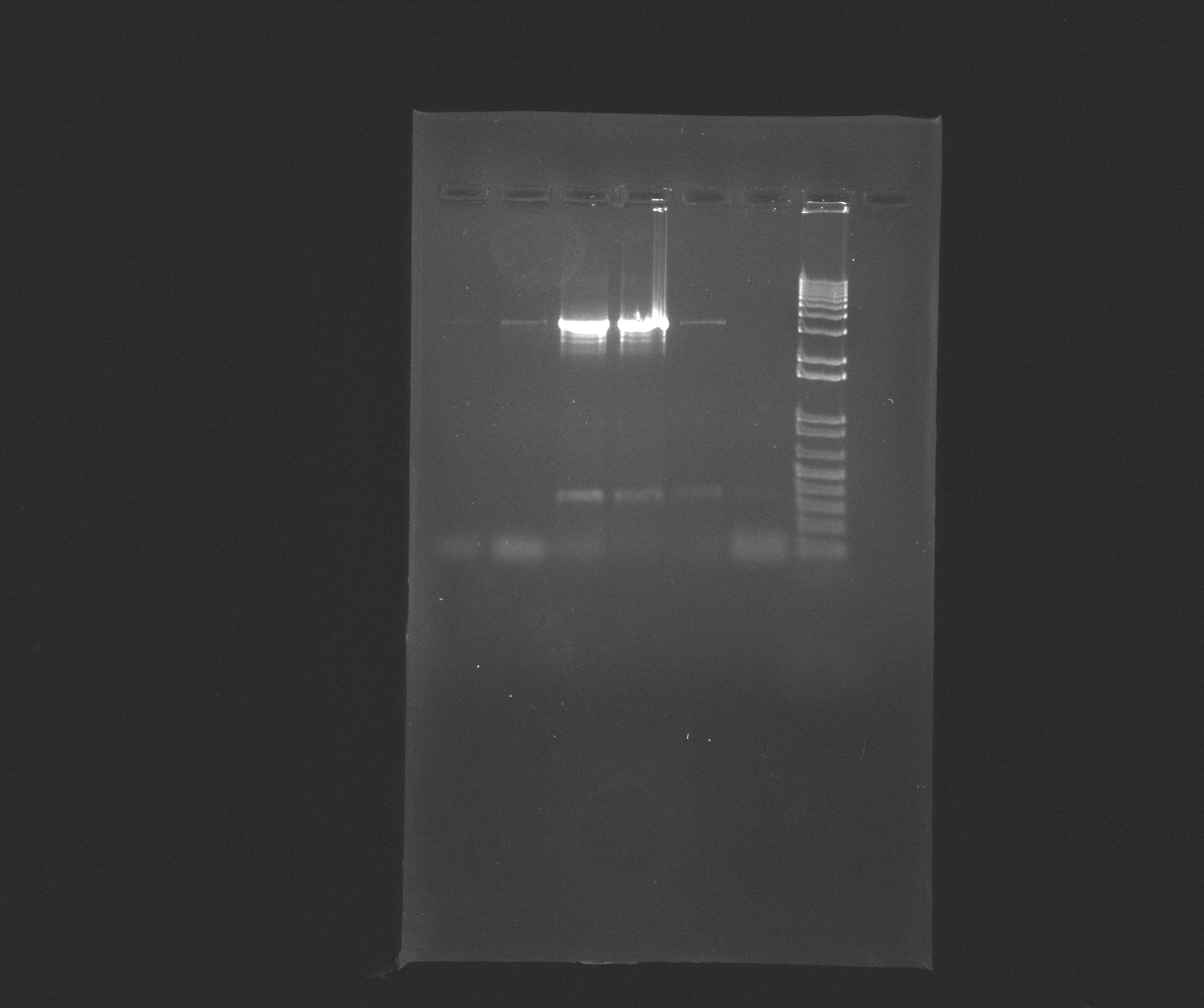
Pictures from gels
Gel AMO
Wells:
- 1: 1 AMO
- 2: 1 AMO
- 3: 10 AMO
- 4: 10 AMO
- 5: 20 AMO
- 6: 20 AMO
- 7: 1 Kb Plus DNA Ladder
Conclusions
Conclusion for AMO
The bands for 10 AMO are thick and it is not visible how many base-pairs they have, so gel extraction and purification is needed.
Gel HAO-CYC
Wells:
- 1: 1 Kb Plus DNA Ladder
- 2: 3 HAO
- 3: 6 HAO
- 4: 10 HAO
- 5: 3 HAO
- 6: 6 HAO
- 7: 10 HAO
Around 3000 base-pairs
- 8: 3 CYC
- 9: 6 CYC
- 10: 10 CYC
- 11: 3 CYC
- 12: 6 CYC
- 13: 10 CYC
Around 1650 base-pairs
- 14: 1 Kb Plus DNA Ladder
Conclusion for HAO and CYC
The bands have shown that the results are close to 2866 bp for HAO and close to 1497 bp for CYC.
Gel with purified AMO,HAO and CYC
Wells
- 8: 1 Kb Plus DNA Ladder
- 11: purified AMO
(Around 3141 bps)
- 12: purified HAO
(Around 2866 bps)
- 13: purified CYC
(Around 1467 bps)
Conclusion for Nir
We clearly see band - not the best but not the worst either. The band are over 12kb according to the ladder which does not match up with the around 9102 we expected from the primer design. We rand a blast on the primers to see if there where other possible binding sites in the chromosome. No good match was found so we purified and will run the purification on a new gel.
Navigate to the Previous or the Next Entry
 "
"