Team:TU Darmstadt/modelling/Structure
From 2013.igem.org
| Line 375: | Line 375: | ||
<body> | <body> | ||
| + | <font size="3" color="#F0F8FF" face="Arial regular"> | ||
| + | <p text-aligne:center style="margin-left:50px; margin-right:50px"> | ||
We present the best three models from a total of 14 listed in the model ranking and sorted by their overall quality Z-scores. | We present the best three models from a total of 14 listed in the model ranking and sorted by their overall quality Z-scores. | ||
<br> | <br> | ||
| Line 384: | Line 386: | ||
</p> | </p> | ||
</body> | </body> | ||
| - | + | <body> | |
| + | <font size="3" color="#F0F8FF" face="Arial regular"> | ||
| + | <p text-aligne:center style="margin-left:50px; margin-right:50px"> | ||
| + | Since the target sequence was the only available information, possible templates were identified by running 6 PSI-BLAST iterations to extract a position specific scoring matrix (PSSM) from Uniprot, and then searching the PDB for a match (i.e. hits with an E-value below the homology modeling cutoff 0.5) | ||
<br> | <br> | ||
<br> | <br> | ||
Revision as of 17:21, 4 October 2013
Homology Modelling
While our proteins are functionally described in literature and during the IGEM competition, only part of the structures are available in the protein data bank. For further work and visualizations, protein structures are indispensable. We used Yasara Structure [1] to calculate 3-dimensional structures of all of our proteins for the IGEM.
Workflow
Description how our Yasara script calculates homology model[7]:

- Sequence is PSI-BLASTed against Uniprot [2]
- Calculation of a position-specific scoring matrix (PSSM) from related sequences
- Using the PSSM to search the PDB for potential modeling templates
- The Templates are ranked based on the alignment score and the structural quality[3]
- Deriving additional information’s for template and target (prediction of secondary structure, structure-based alignment correction by using SSALN scoring matrices [4]).
- A graph of the side-chain rotamer network is built, dead-end elimination is used to find an initial rotamer solution in the context of a simple repulsive energy function [5]
- The loop-network is optimized using a high amount of different orientations
- Side-chain rotamers are fine-tuned considering electrostatic and knowledge-based packing interactions as well as solvation effects.
- An unrestrained high-resolution refinement with explicit solvent molecules is run, using the latest knowledge-based force fields[6].
Application
All these steps are performed to every template used for the modeling approach. For our project we set the maximum amount of templates to 20. Every derived structure is evaluated using an average per-residue quality Z-scores. At last a hybrid model is built containing the best regions of all predictions. This procedure make prediction’s accurate and thus more realistic. For the evaluation we used the Yasara Z-scores.A Z-score describes how many standard deviations the model quality is away from the average high-resolution X-ray structure. Negative values indicate that the homology model looks worse than a high-resolution X-ray structure. The overall Z-scores for all models have been calculated as the weighted averages of the individual Z-scores using the formula Overall = 0.145*Dihedrals + 0.390*Packing1D + 0.465*Packing3D [7].
Parameters
We used the Yasara script hm_build.mcr for the model creation with the following parameters:
- Modeling speed (slow = best): Slow
- Number of PSI-BLAST iterations in template search (PsiBLASTs): 3
- Maximum allowed PSI-BLAST E-value to consider template (EValue Max): 0.5
- Maximum number of templates to be used (Templates Total): 20
- Maximum number of templates with same sequence (Templates SameSeq): 1
- Maximum oligomerization state (OligoState): 4 (tetrameric)
- Maximum number of alignment variations per template: (Alignments): 5
- Maximum number of conformations tried per loop (LoopSamples): 50
- Maximum number of residues added to the termini (TermExtension): 10
Results
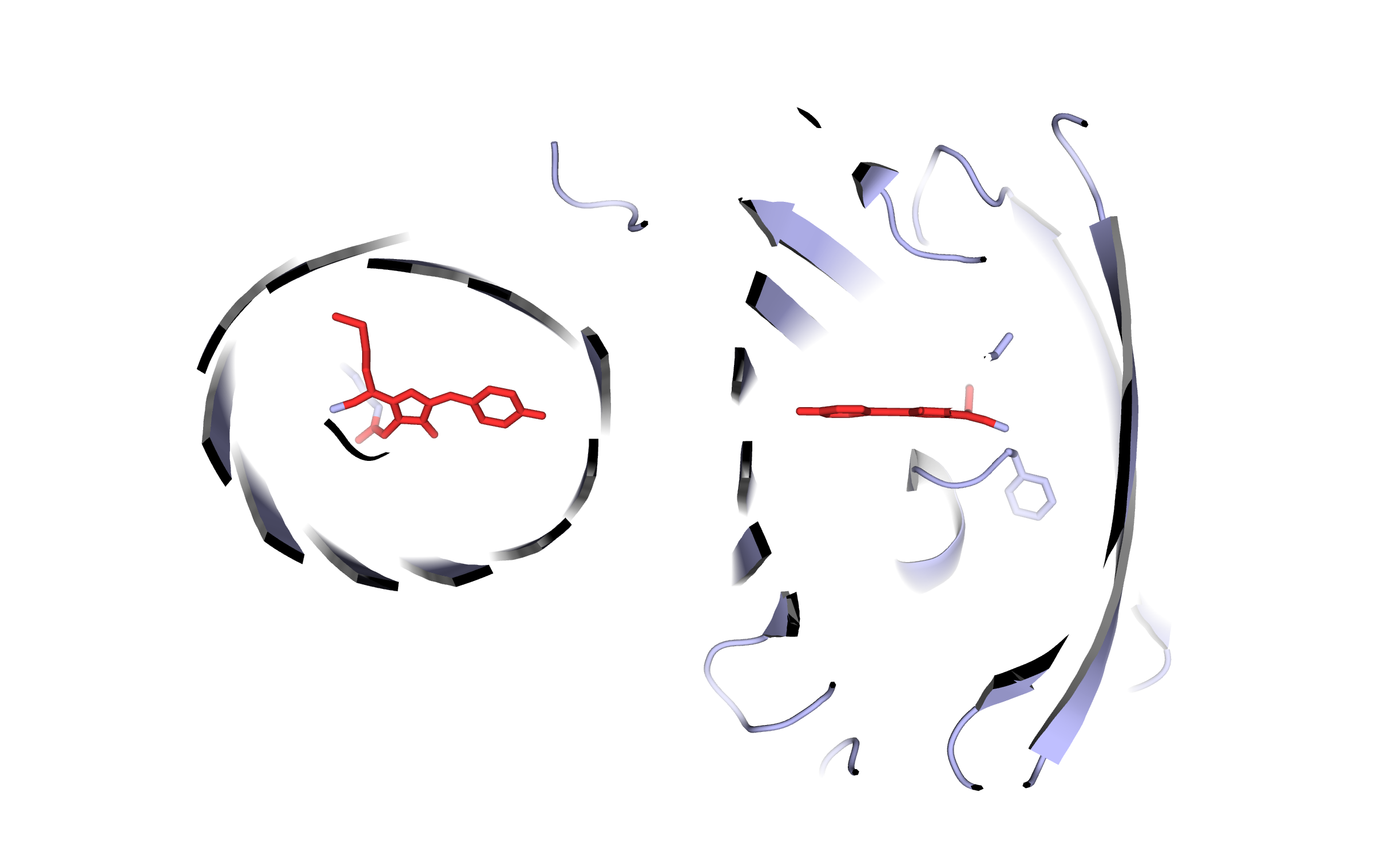
We present the best three models from a total of 14 listed in the model ranking and sorted by their overall quality Z-scores.
First model LssmOrange
Since the target sequence was the only available information, possible templates were identified by running 6 PSI-BLAST iterations to extract a position specific scoring matrix (PSSM) from Uniprot, and then searching the PDB for a match (i.e. hits with an E-value below the homology modeling cutoff 0.5)
LssmOrange - second modelling
Third model LssmOrange (dimer)
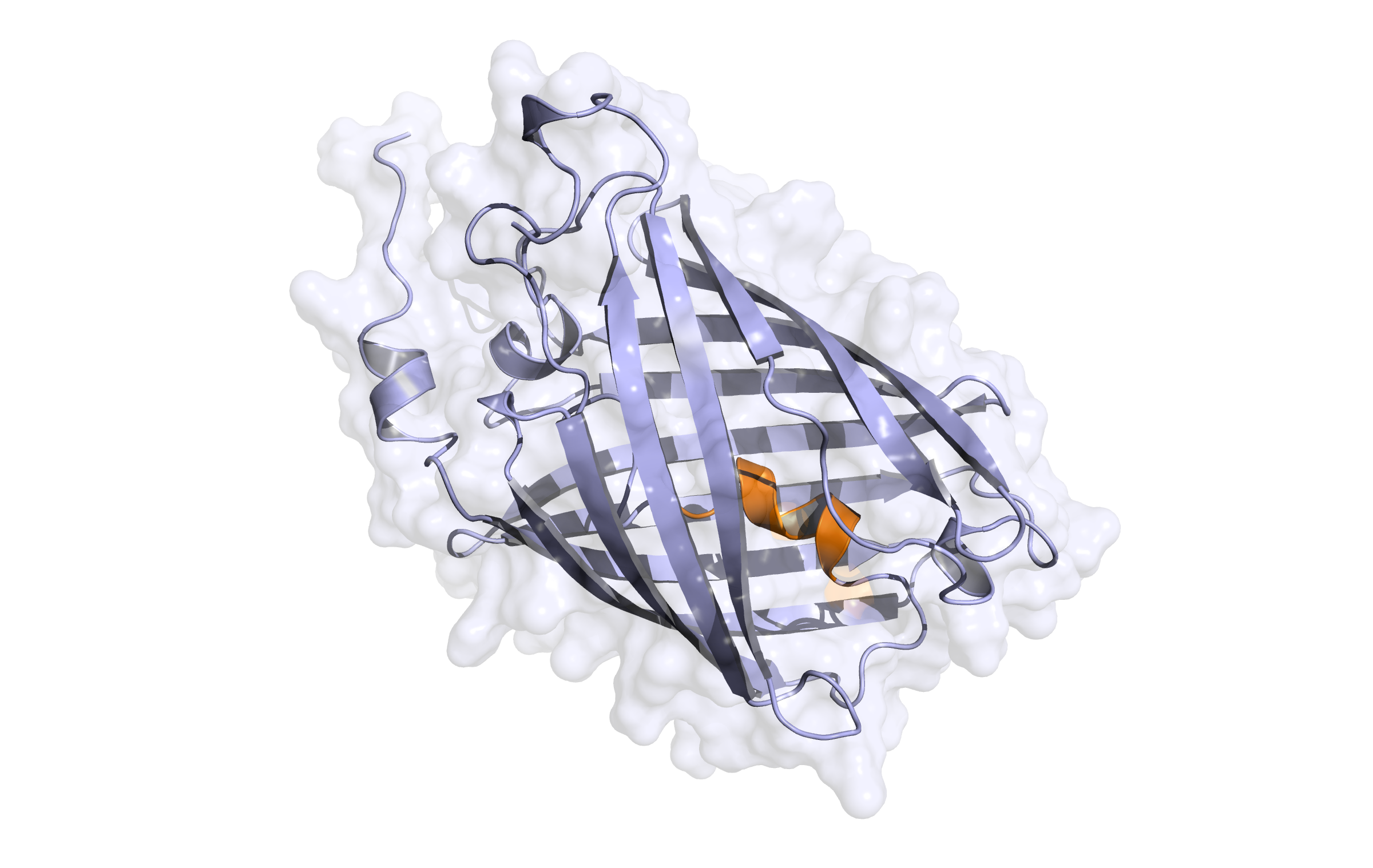

Here, the hybrid homology model of our protein,
LssmOrange
is shown in ribbon representation. Furthermore, we illustrate the average quality z-score as a function of residue number. Nevertheless, it was subjected to a final round of simulated annealing minimization in explicit solvent and obtained the following quality Z-scores:
Check Type
Quality Z-score
Comment
Dihedrals
2.363
Optimal
Packing 1D
-0.864
Good
Packing 3D
-1.138
Satisfactory
Overall
-0.524
Good
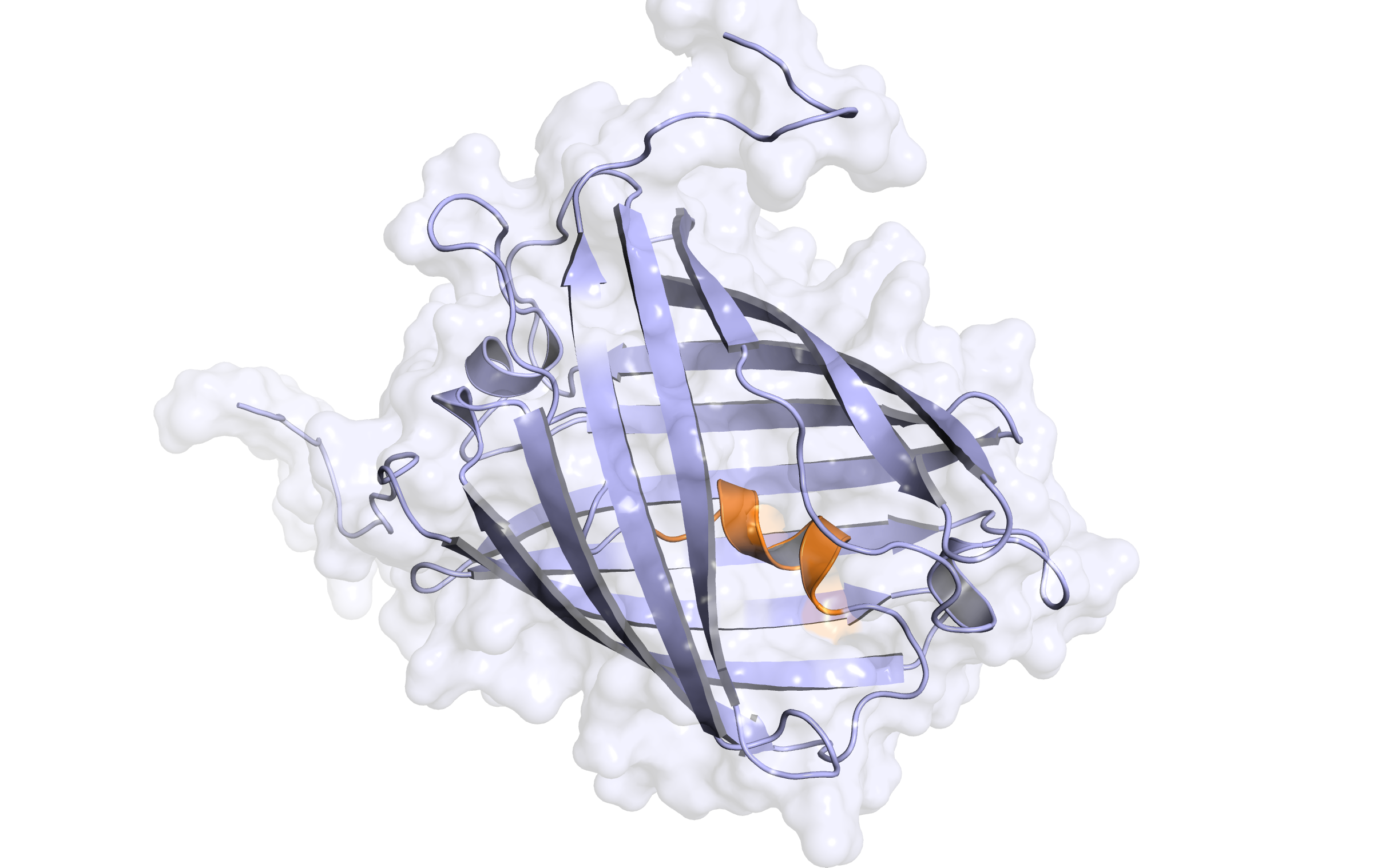

Check Type
Quality Z-score
Comment
Dihedrals
2.125
Optimal
Packing 1D
-0.936
Good
Packing 3D
-1.259
Satisfactory
Overall
-0.642
Good
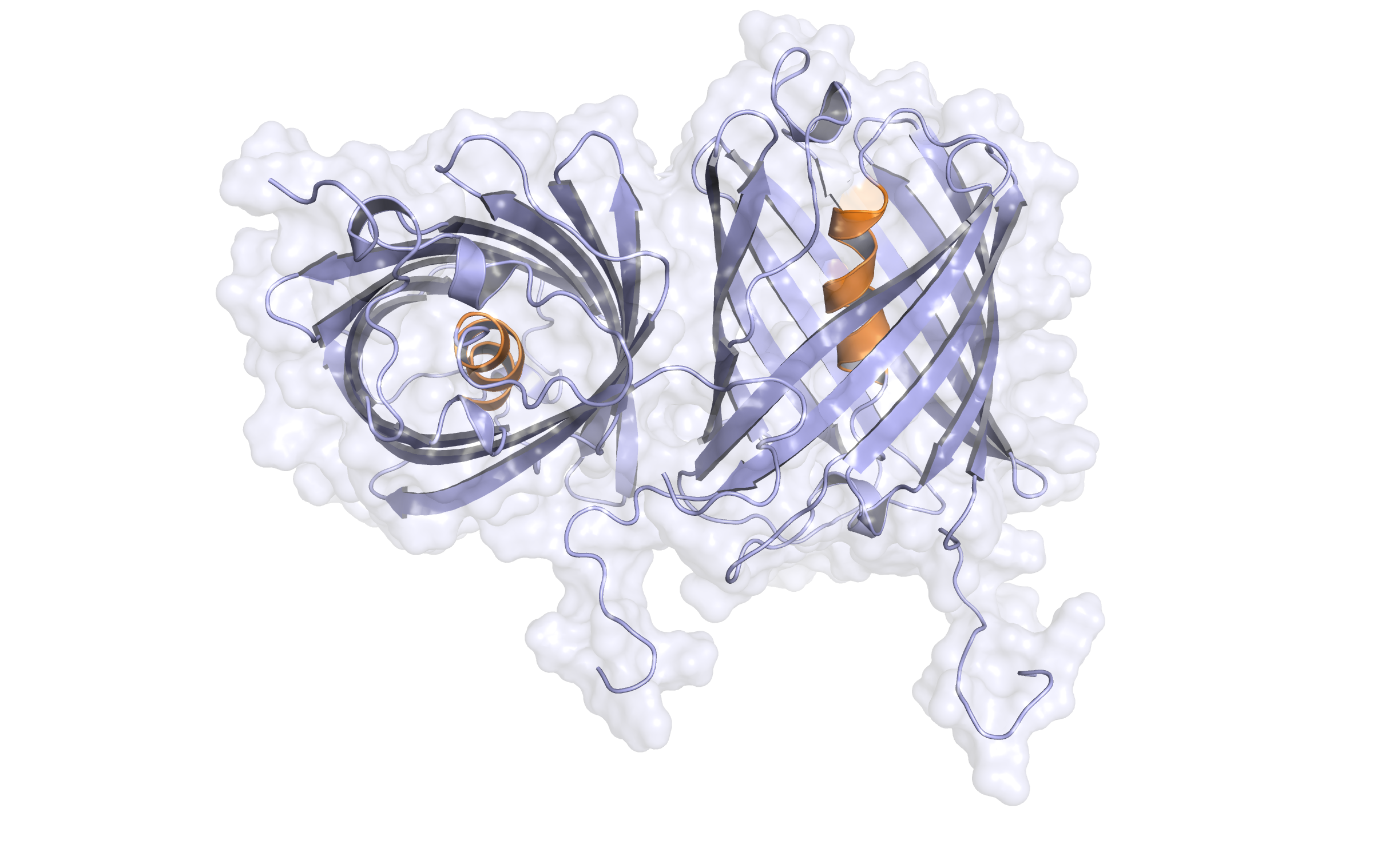

Check Type
Quality Z-score
Comment
Dihedrals
1.237
Optimal
Packing 1D
-2.083
Poor
Packing 3D
-1.651
Satisfactory
Overall
-1.400
Satisfactory
References
[1] E. Krieger, G. Koraimann, and G. Vriend, “Increasing the precision of comparative models with YASARA NOVA--a self-parameterizing force field.,” Proteins, vol. 47, no. 3, pp. 393–402, 2002.
[2] S. F. Altschul, T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman, “Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.,” Nucleic Acids Res, vol. 25, no. 17, pp. 3389–3402, Sep. 1997.
[3] R. W. Hooft, G. Vriend, C. Sander, and E. E. Abola, “Errors in protein structures.,” Nature, vol. 381, no. 6580. Nature Publishing Group, p. 272, 1996.
[4] D. T. Jones, “Protein secondary structure prediction based on position-specific scoring matrices,” Journal of Molecular Biology, vol. 292, no. 2, pp. 195–202, 1999.
[5] A. A. Canutescu, A. A. Shelenkov, and R. L. Dunbrack, “A graph-theory algorithm for rapid protein side-chain prediction.,” Protein Science, vol. 12, no. 9, pp. 2001–2014, 2003.
[6] E. Krieger, K. Joo, J. Lee, J. Lee, S. Raman, J. Thompson, M. Tyka, D. Baker, and K. Karplus, “Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8.,” Proteins, vol. 77 Suppl 9, no. June, pp. 114–122, 2009.
[7] http://www.yasara.org/homologymodeling.htm
 "
"