Team:BYU Provo/Notebook/LargePhage/Springexp/Period3/PR
From 2013.igem.org
| Line 1: | Line 1: | ||
{{TeamBYUProvo}} | {{TeamBYUProvo}} | ||
PROGRESS REPORT | PROGRESS REPORT | ||
| - | === | + | ===Accomplishments=== |
| - | + | Began Mutagenesis with UV light | |
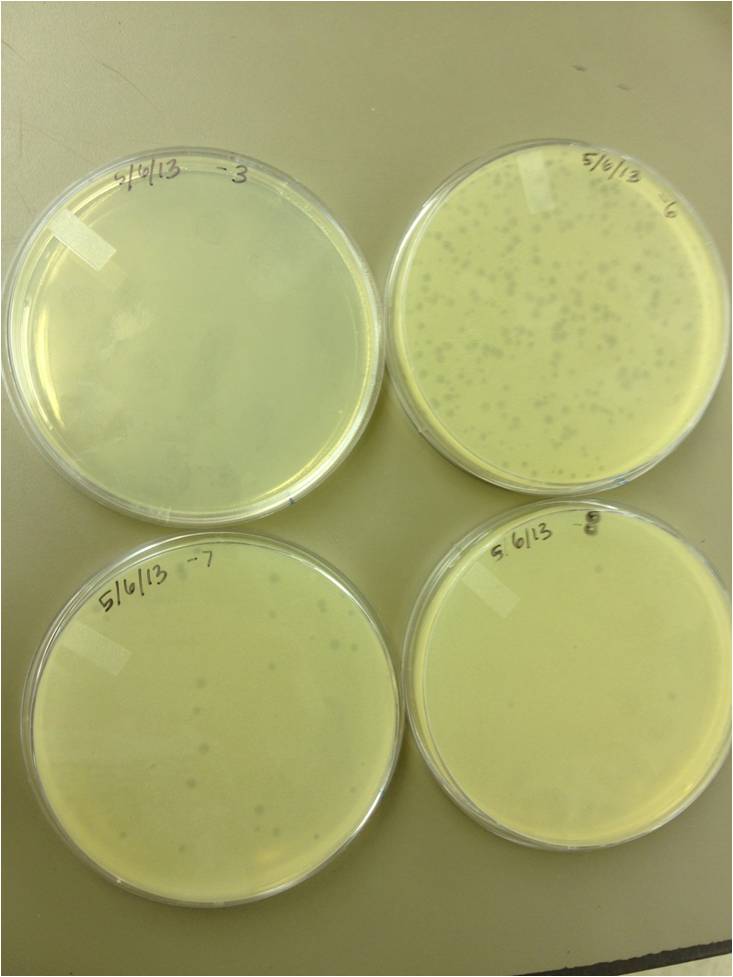
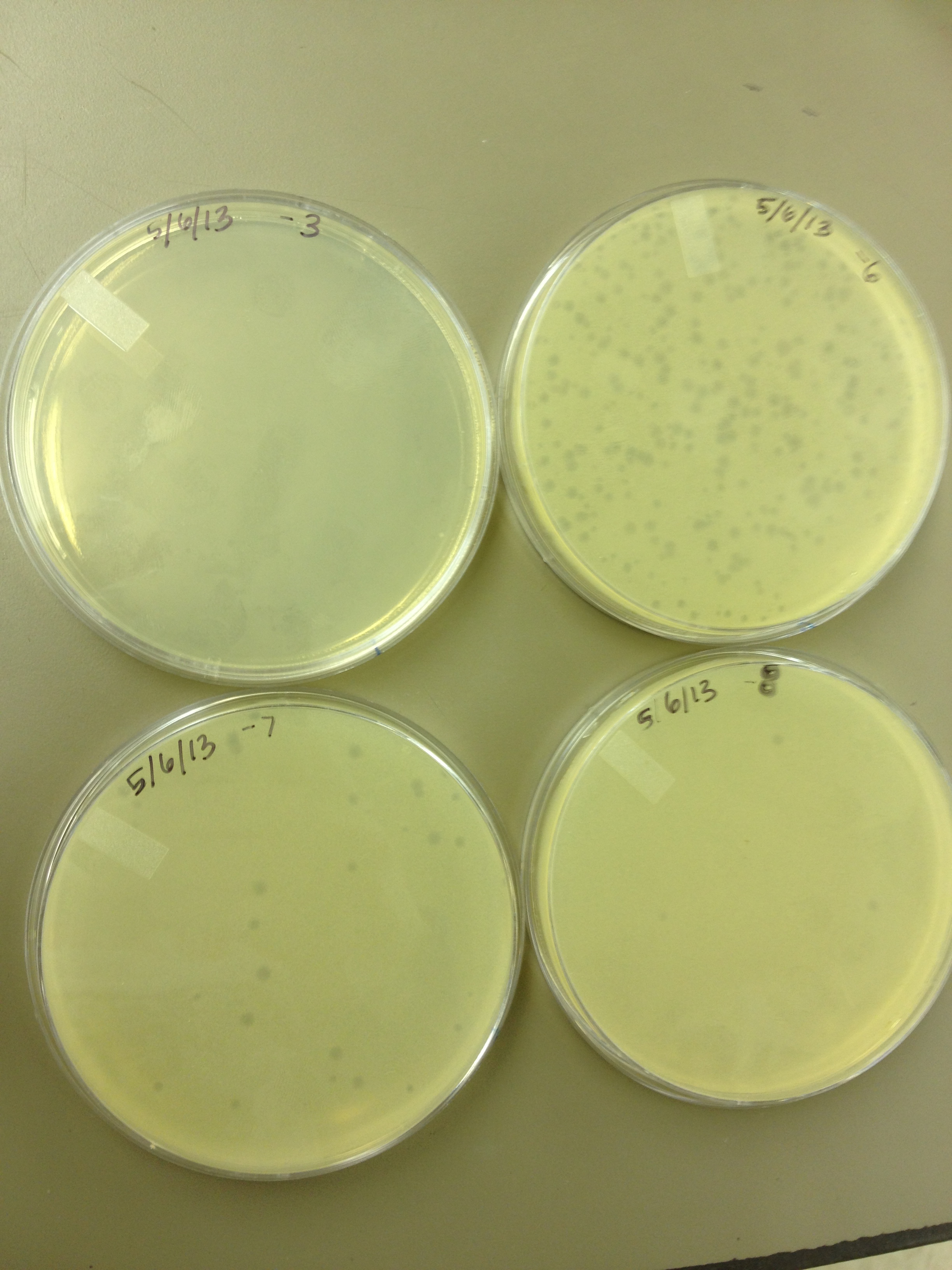
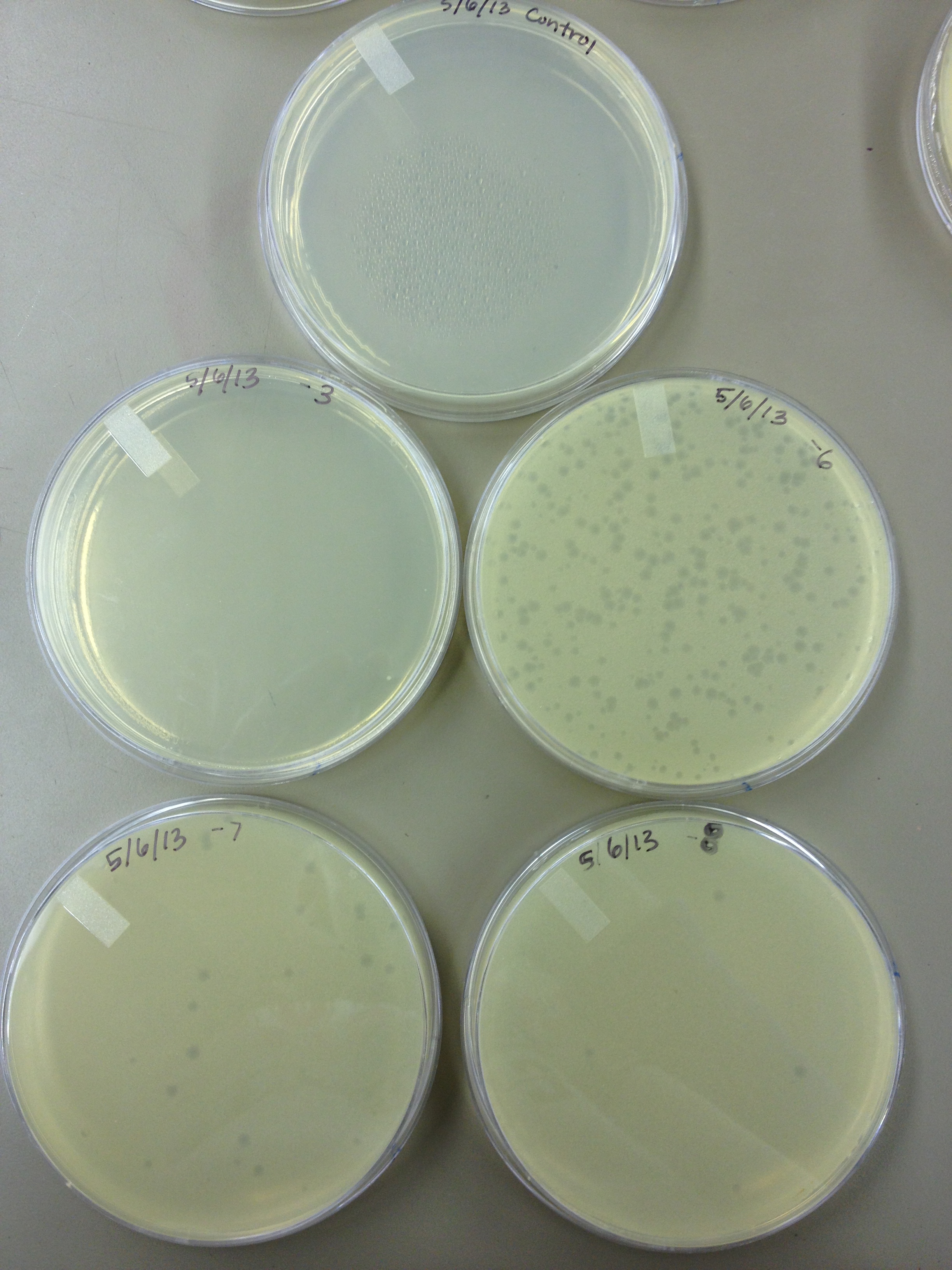
| - | Today we | + | Today we did a phage titer of 0,-3,-6,-7,-8,-9,-10,-11 We started by putting 1 ul of phage into 1ml of bacteria, we went down from there. We infected each test tube with 10 uL from each dilution level. |
| - | + | ||
| - | + | ||
| - | 10 | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
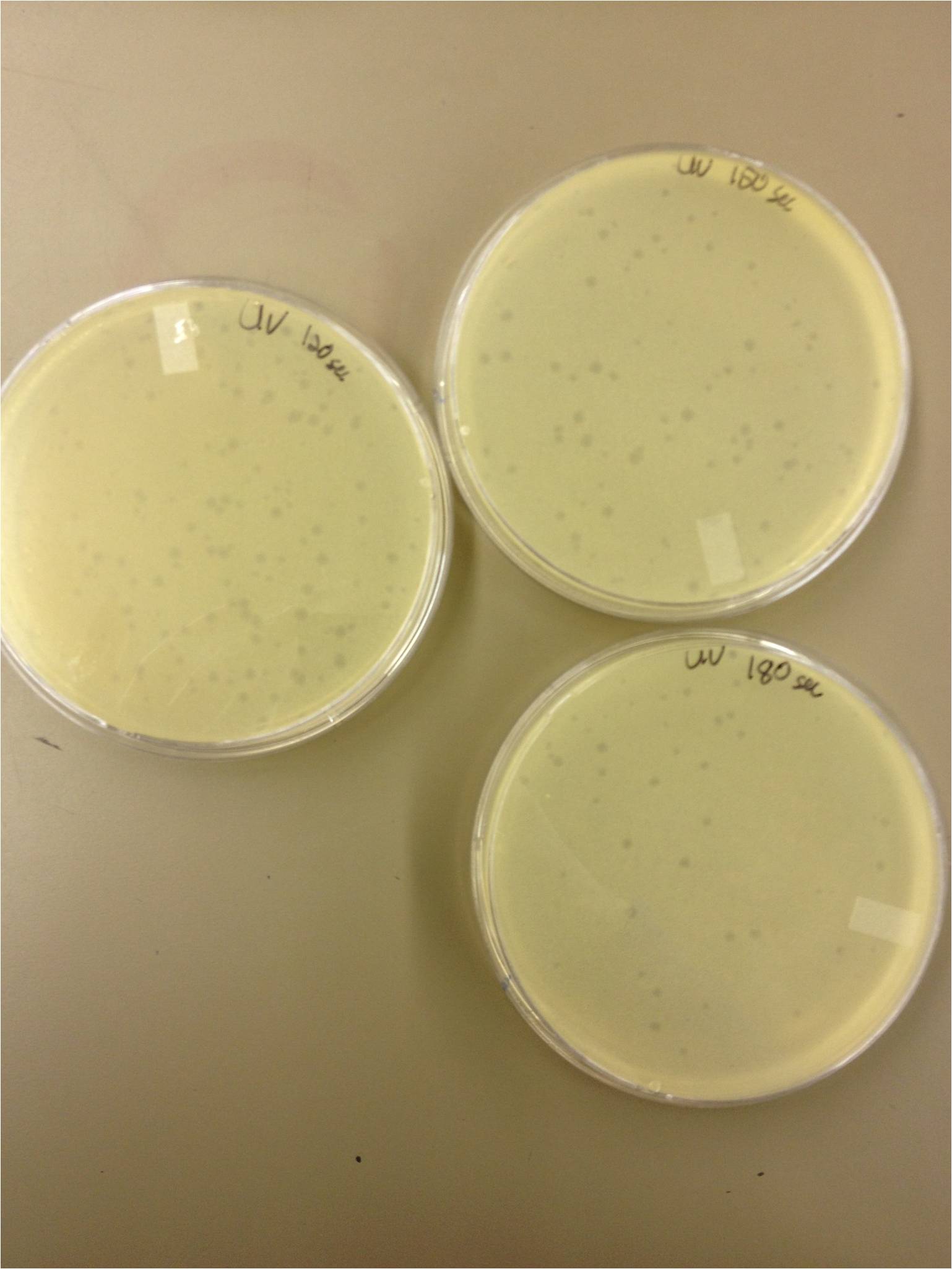
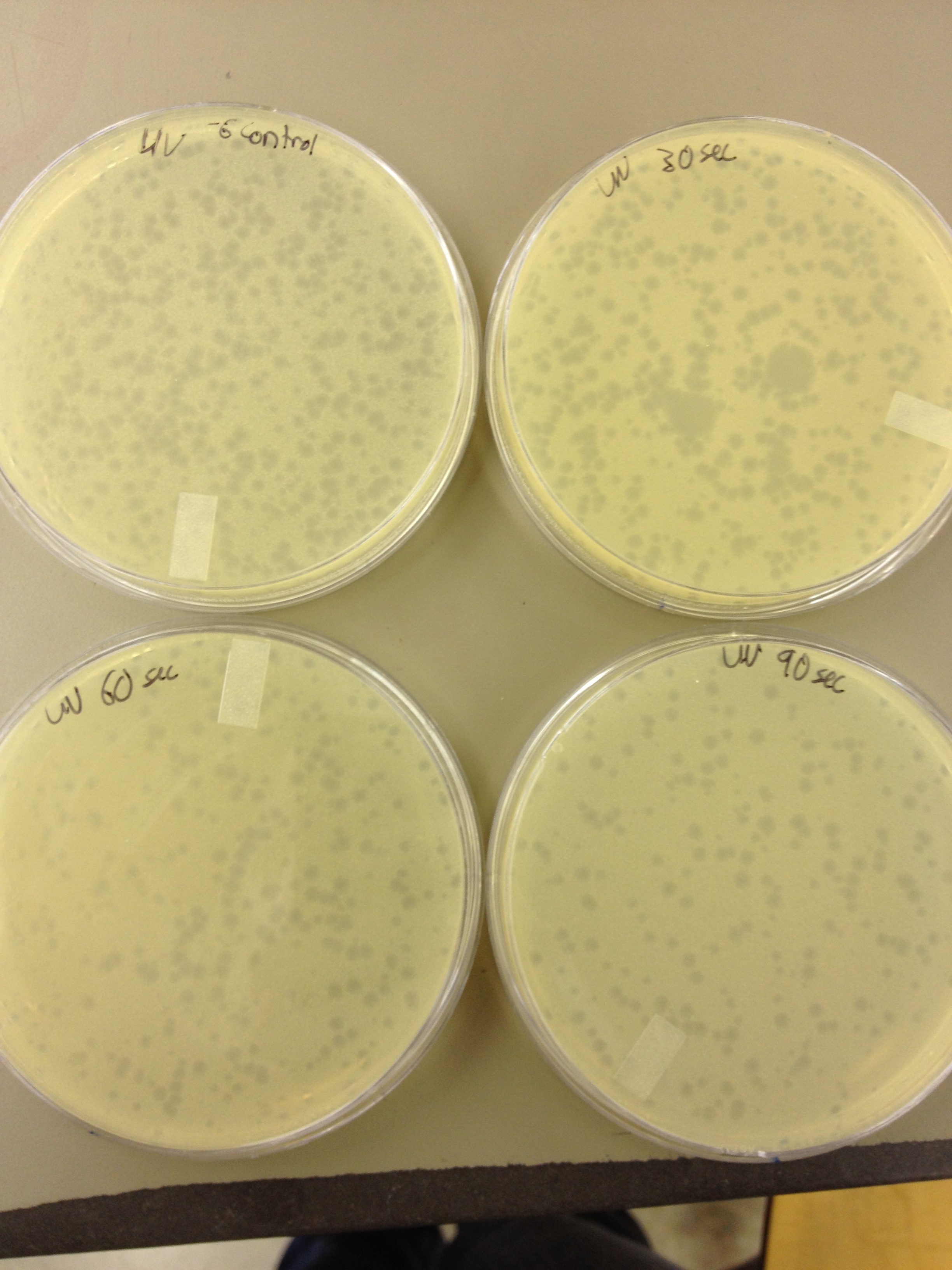
| - | + | We also did a UV pre-test of sorts. We put 20ul of phage onto parafilm and exposed it to UV light in the hood of Dr. Breakwell’s lab. At 30 second intervals we took the 20 ul’s off and put it in a half ml of e.coli W3110. We tested every 30 seconds up to 3 minutes. | |
| - | + | ||
| + | We also streaked out the W3110 bacteria from freezer stock onto an LB plate so we can use it to start future bacterial cultures. | ||
| + | |||
| + | [[File:Ex.jpg|400px|center|]] | ||
| + | [[File:Ex1.jpg|400px|center|]] | ||
| + | [[File:Ex2.jpg|400px|center|]] | ||
| + | [[File:Ex3.jpg|400px|center|]] | ||
| + | |||
| + | ===5/8/13=== | ||
| + | 5/8/13- Results from Monday KS BDM | ||
| + | |||
| + | [[File:hey1.jpeg|400px|thumb|center|]] | ||
| + | [[File:hey2.jpeg|400px|thumb|center|]] | ||
| + | [[File:hey3.jpeg|400px|thumb|center|]] | ||
| + | [[File:hey4.jpeg|400px|thumb|center|]] | ||
| + | |||
| + | We looked at the results for the dilution series (titer) we did on the phage stock we made from the liquid culture and found that there were 5 plaques on the 10^-8 plate. | ||
| + | |||
| + | We calculated pfus/mL by doing ( # of plaques ) / (dilution level x mLs infected with). Our stock is between 3x10^9 and 5x10^9 pfus/mL. | ||
| + | 180 sec = ~50 plaques | ||
| + | 150 sec = ~102 plaques | ||
| + | 120 sec = ~170 plaques | ||
| + | 90 sec = ~280 | ||
Plaques/(dilution * amt infected with) | Plaques/(dilution * amt infected with) | ||
Revision as of 19:17, 7 June 2013
PROGRESS REPORT
Contents |
Accomplishments
Began Mutagenesis with UV light Today we did a phage titer of 0,-3,-6,-7,-8,-9,-10,-11 We started by putting 1 ul of phage into 1ml of bacteria, we went down from there. We infected each test tube with 10 uL from each dilution level.
We also did a UV pre-test of sorts. We put 20ul of phage onto parafilm and exposed it to UV light in the hood of Dr. Breakwell’s lab. At 30 second intervals we took the 20 ul’s off and put it in a half ml of e.coli W3110. We tested every 30 seconds up to 3 minutes.
We also streaked out the W3110 bacteria from freezer stock onto an LB plate so we can use it to start future bacterial cultures.
5/8/13
5/8/13- Results from Monday KS BDM
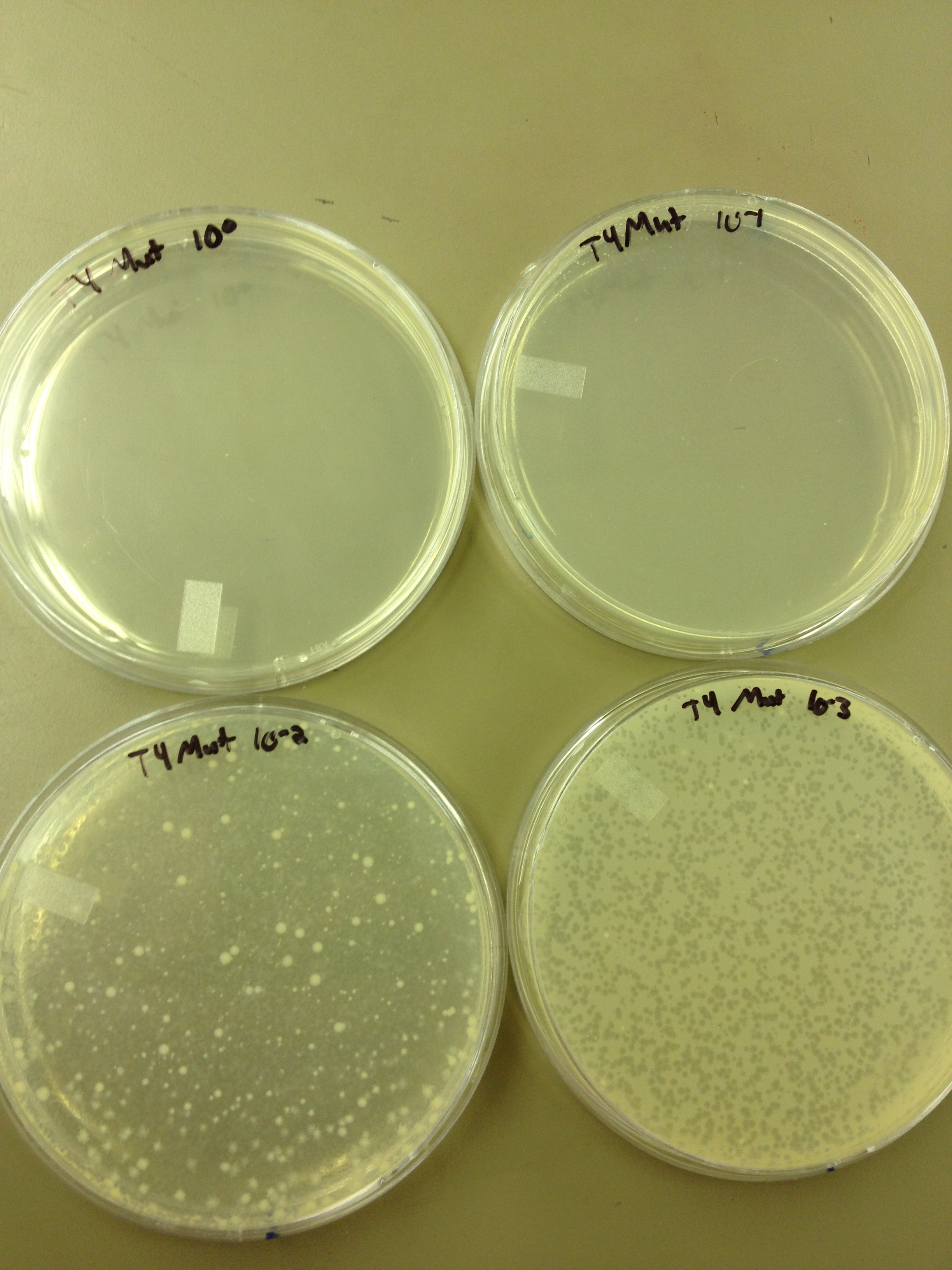
We looked at the results for the dilution series (titer) we did on the phage stock we made from the liquid culture and found that there were 5 plaques on the 10^-8 plate.
We calculated pfus/mL by doing ( # of plaques ) / (dilution level x mLs infected with). Our stock is between 3x10^9 and 5x10^9 pfus/mL. 180 sec = ~50 plaques 150 sec = ~102 plaques 120 sec = ~170 plaques 90 sec = ~280
Plaques/(dilution * amt infected with)
5/17/13
5/20/13
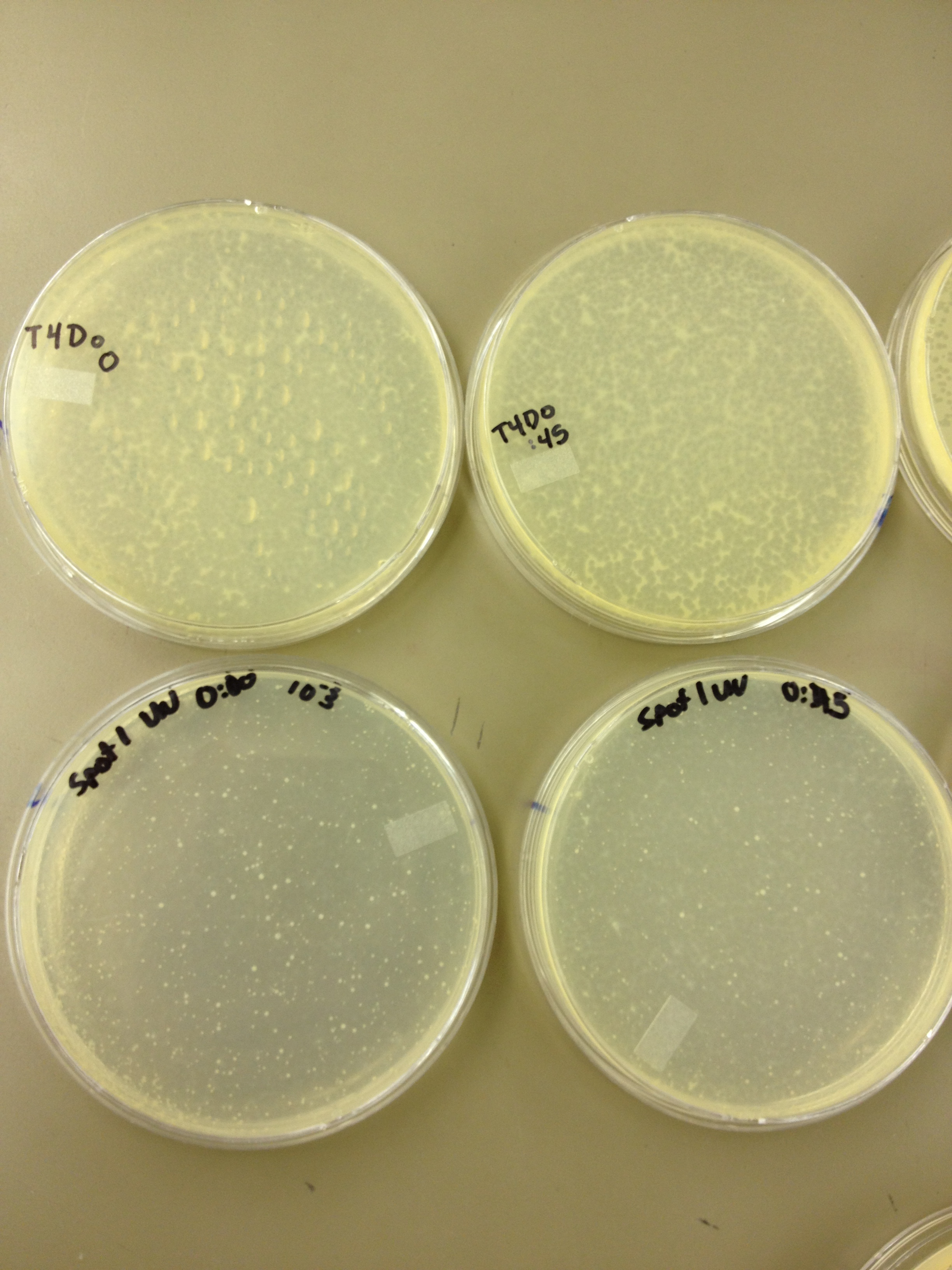
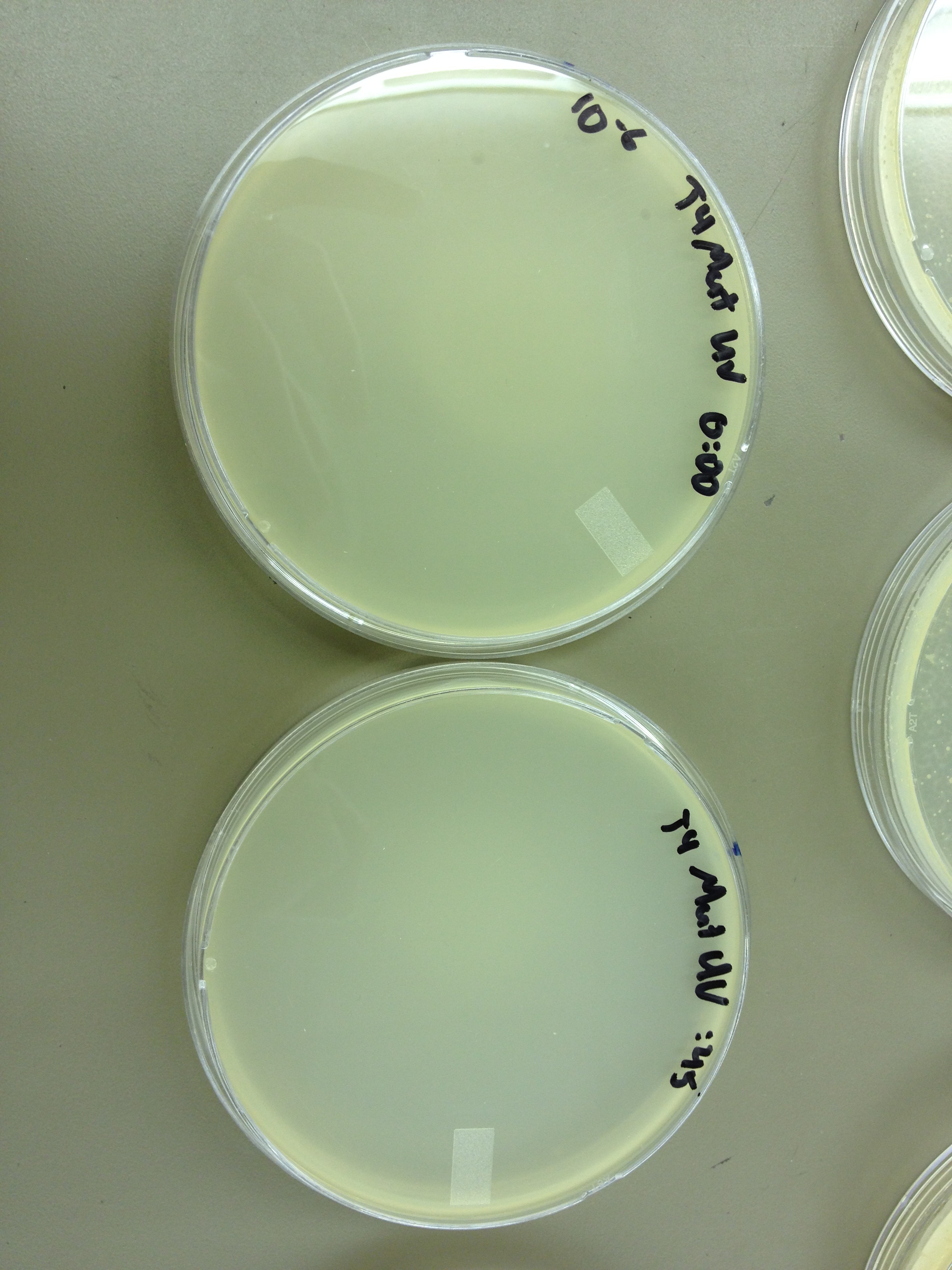
Today we need to run a dilution series to test the titer of our mutated phage stock. We also need to start selecting for small plaques and learning how to pick them and titer them out. We also should run a UV test on the mutated phage stock compared to the normal stock.
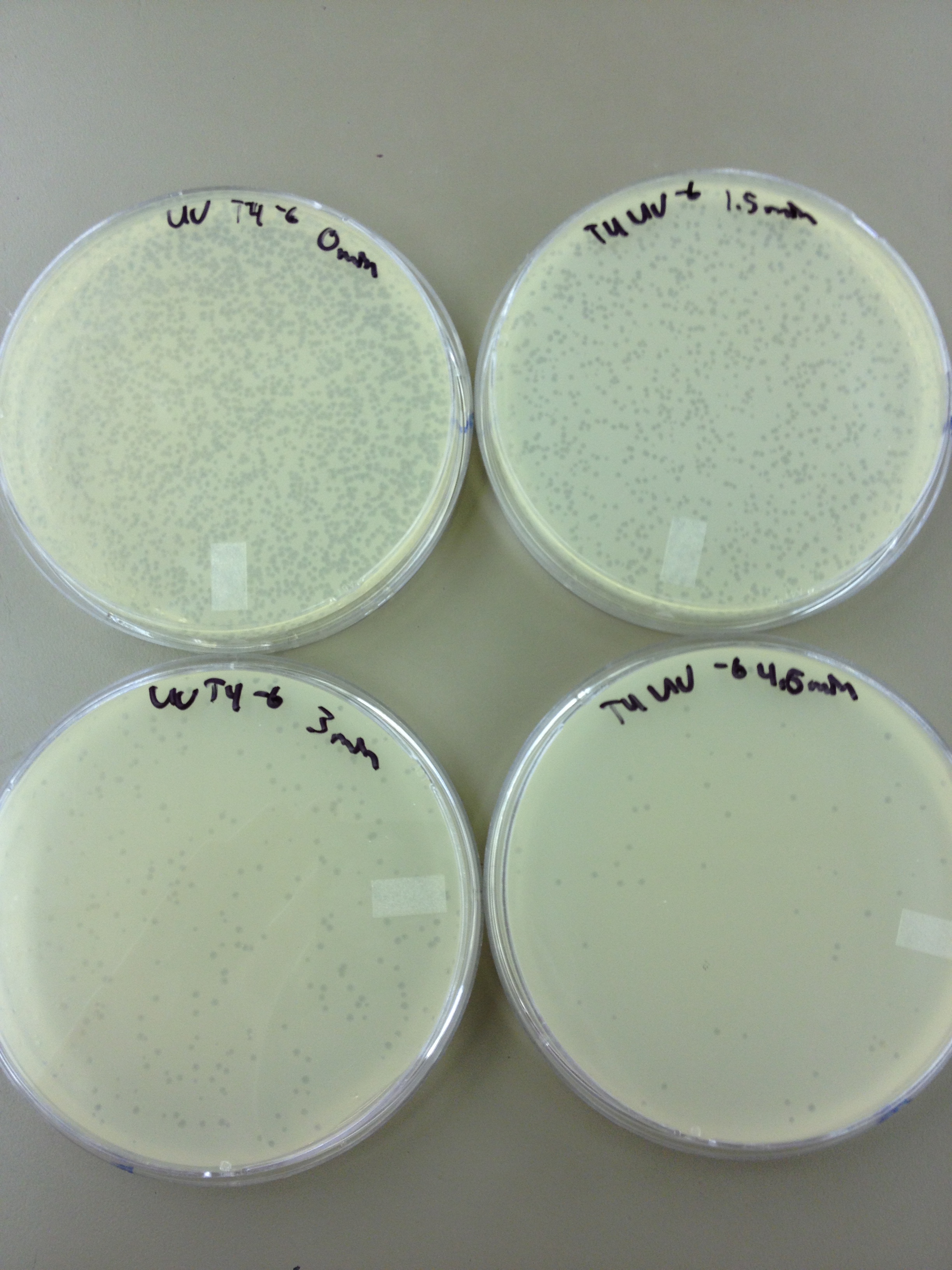
We picked one plaque off of the 180 sec UV plate sample. We suspended it in 1 mL of broth, and then UV-ed 20 uL samples at 45 second intervals. The number of plaques decreased the longer the samples sat under UV light. The samples were irradiated from 0 sec to 4 min 30 sec.
As a control, we diluted the T4Do stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 6 min) under UV light.
Also, we diluted the T4 mutagenized stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 4 min 30 sec) under UV light.
UV tests were done by placing 20 uL spots on parafilm and placed in a BSL-2 hood with the UV light turned on.
5/22/13
Results from 5/20/13
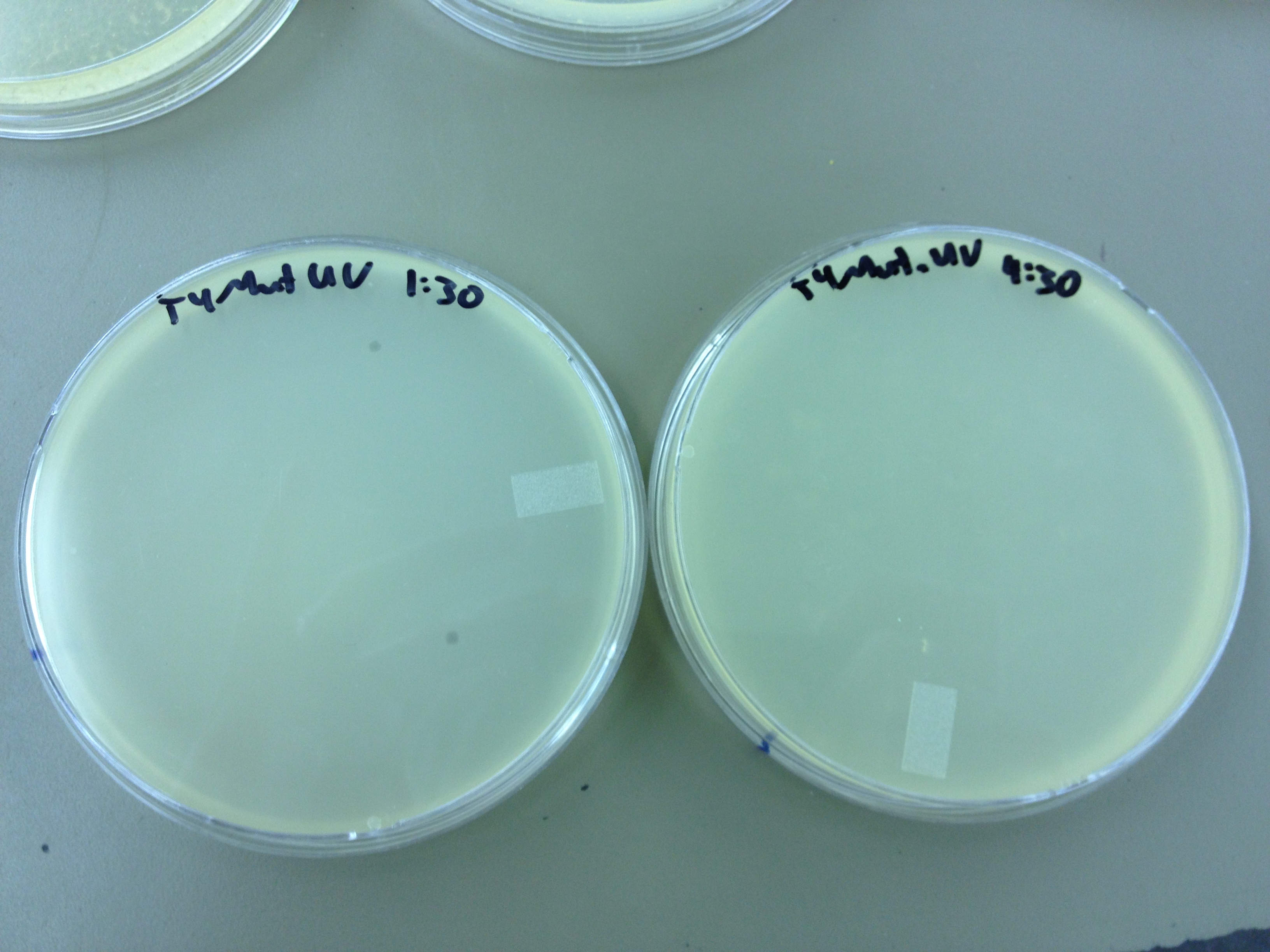
EVERY 1:30 Today we need to run a dilution series to test the titer of our mutated phage stock. We also need to start selecting for small plaques and learning how to pick them and titer them out. We also should run a UV test on the mutated phage stock compared to the normal stock.
We picked one plaque off of the 180 sec UV plate sample. We suspended it in 1 mL of broth, and then UV-ed 20 uL samples at 45 second intervals. The number of plaques decreased the longer the samples sat under UV light. The samples were irradiated from 0 sec to 4 min 30 sec.
As a control, we diluted the T4Do stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 6 min) under UV light.
Also, we diluted the T4 mutagenized stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 4 min 30 sec) under UV light.
UV tests were done by placing 20 uL spots on parafilm and placed in a BSL-2 hood with the UV light turned on.
5/24/13
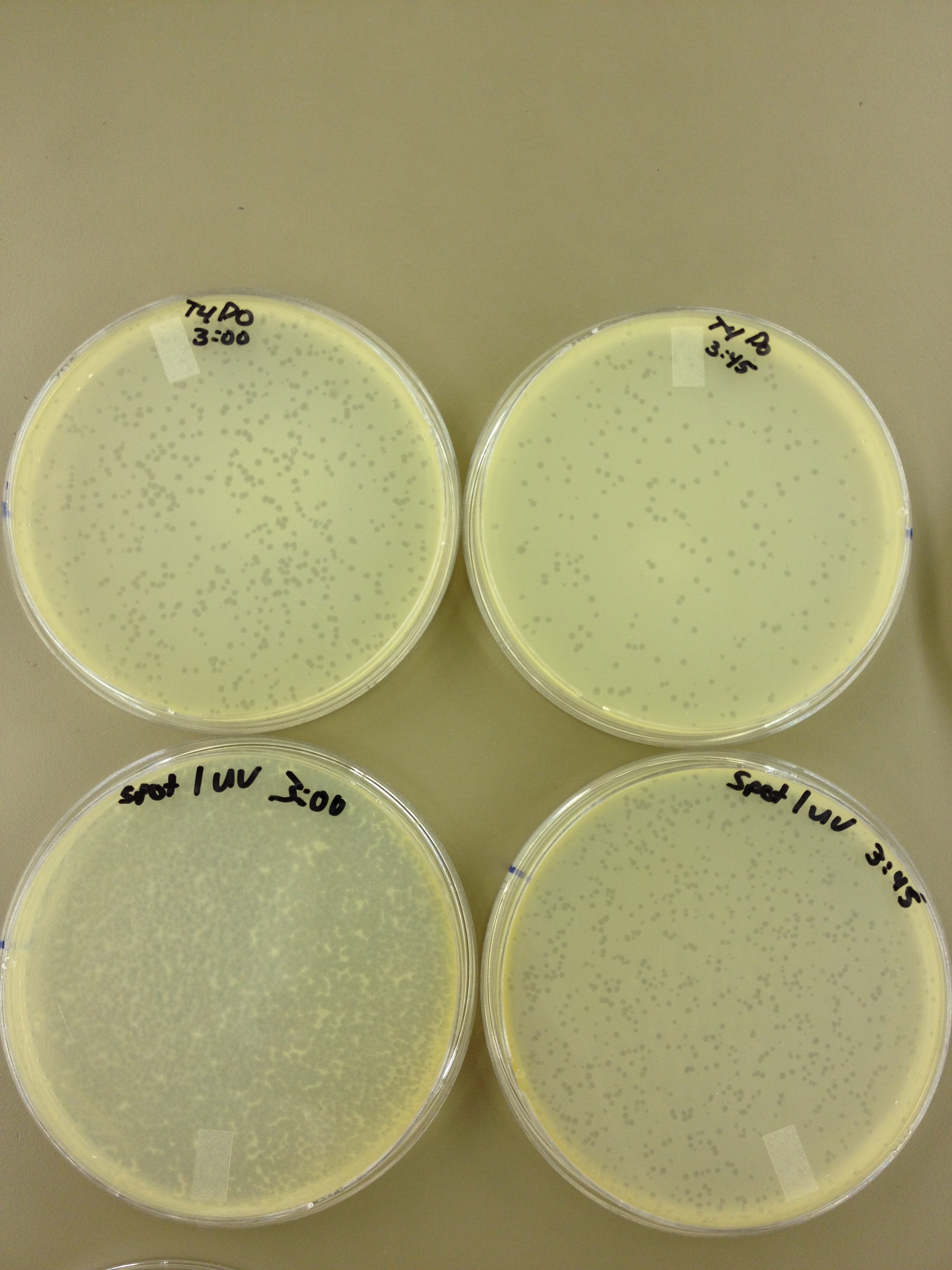
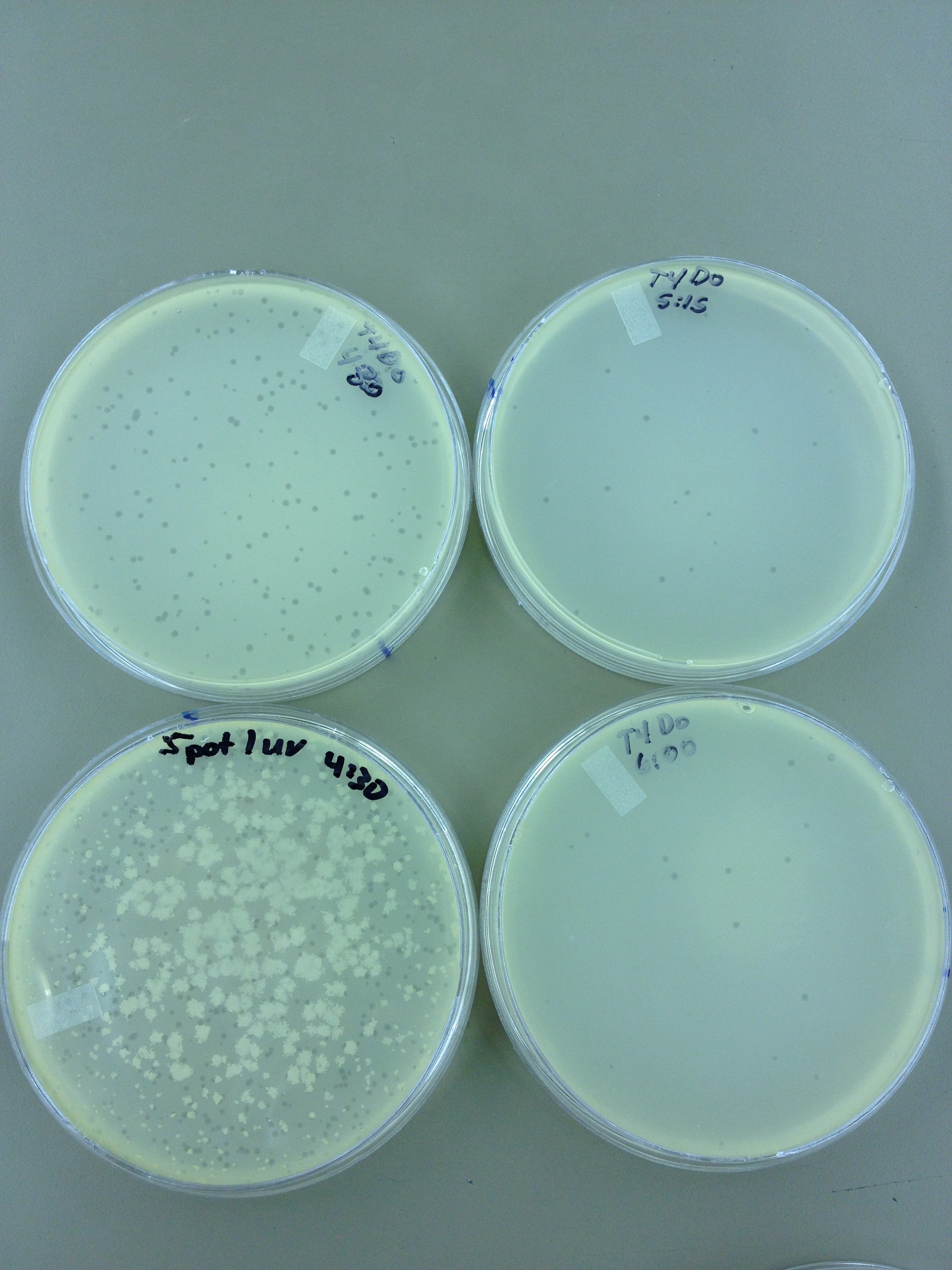
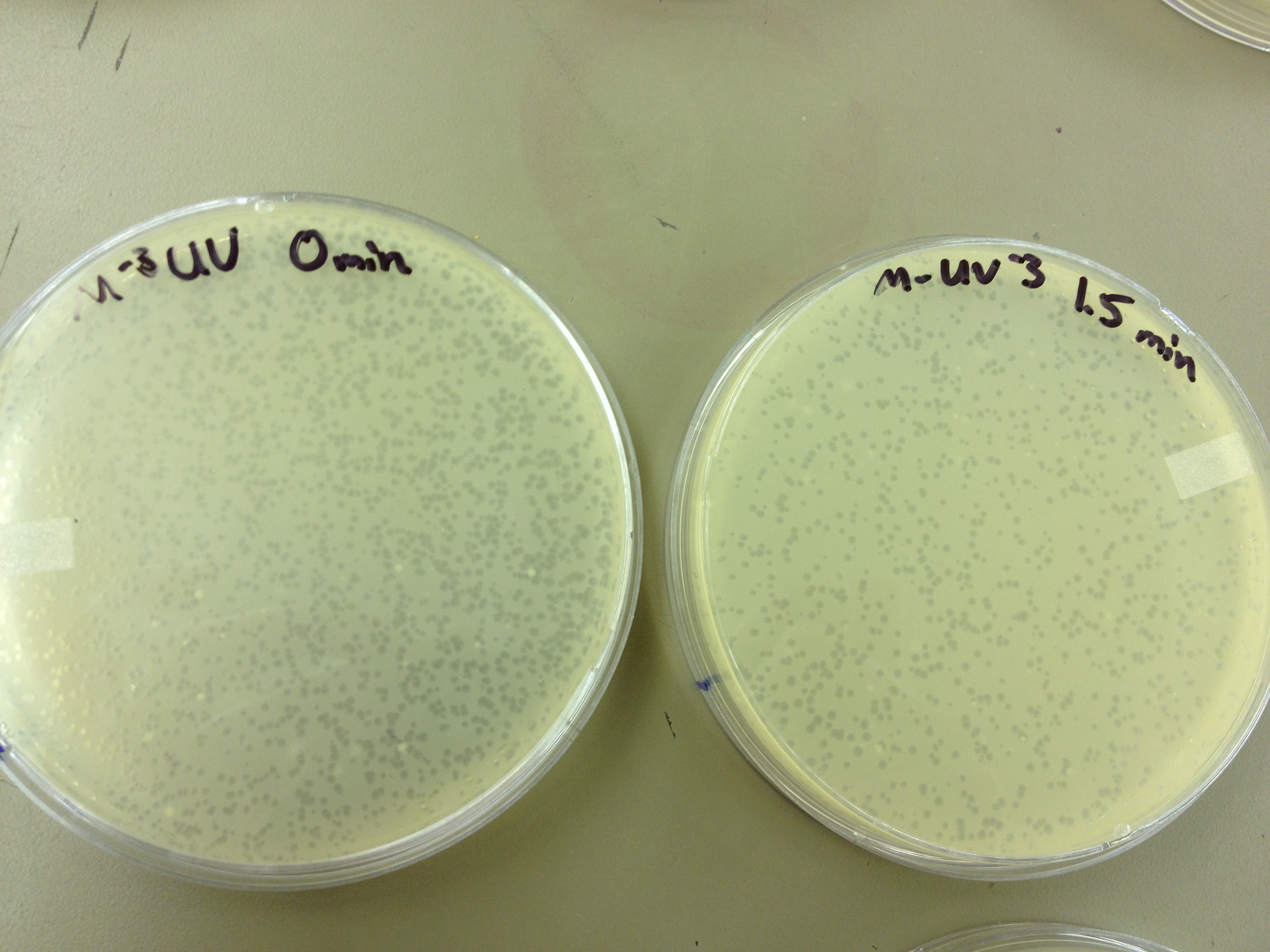
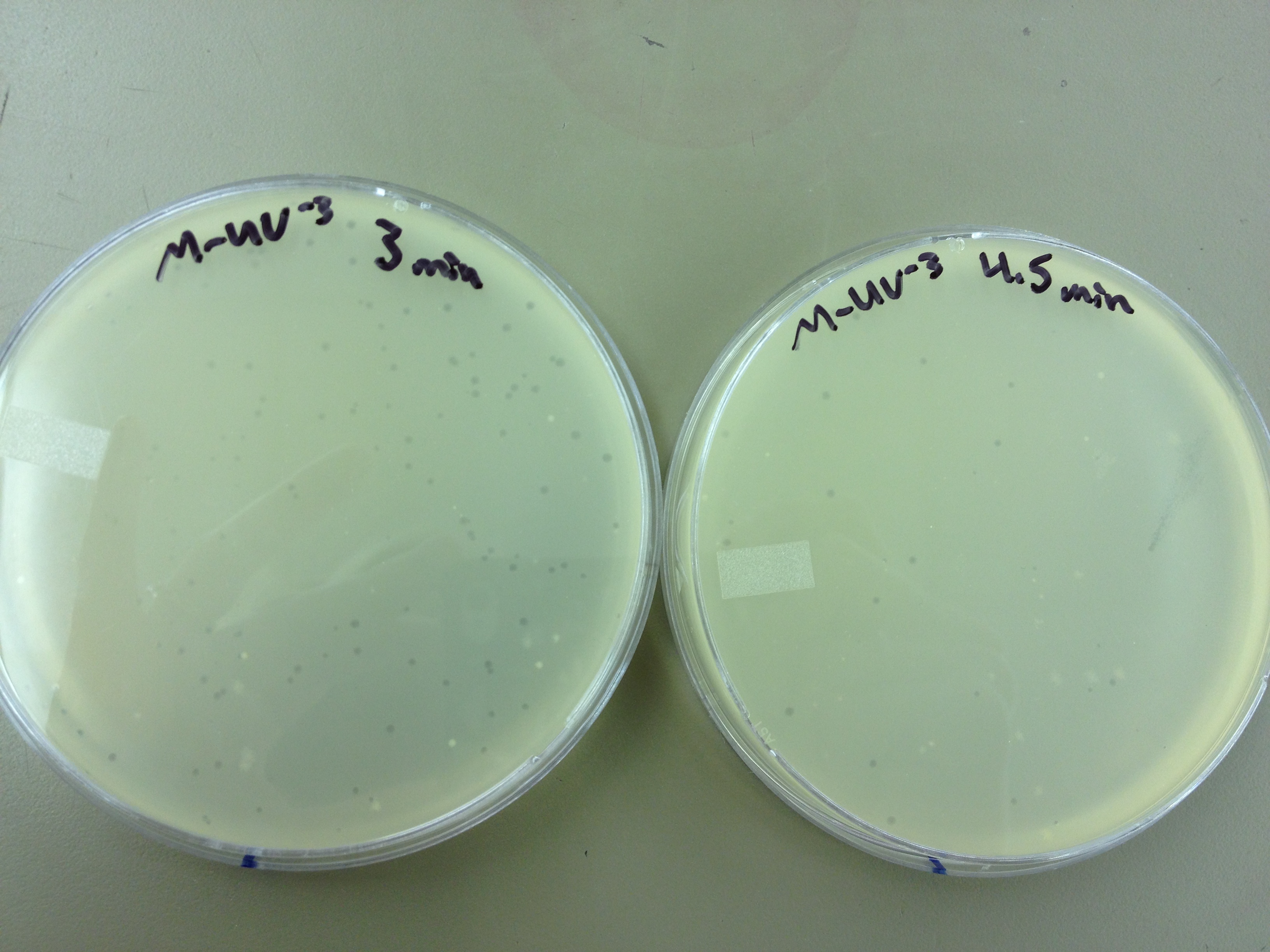
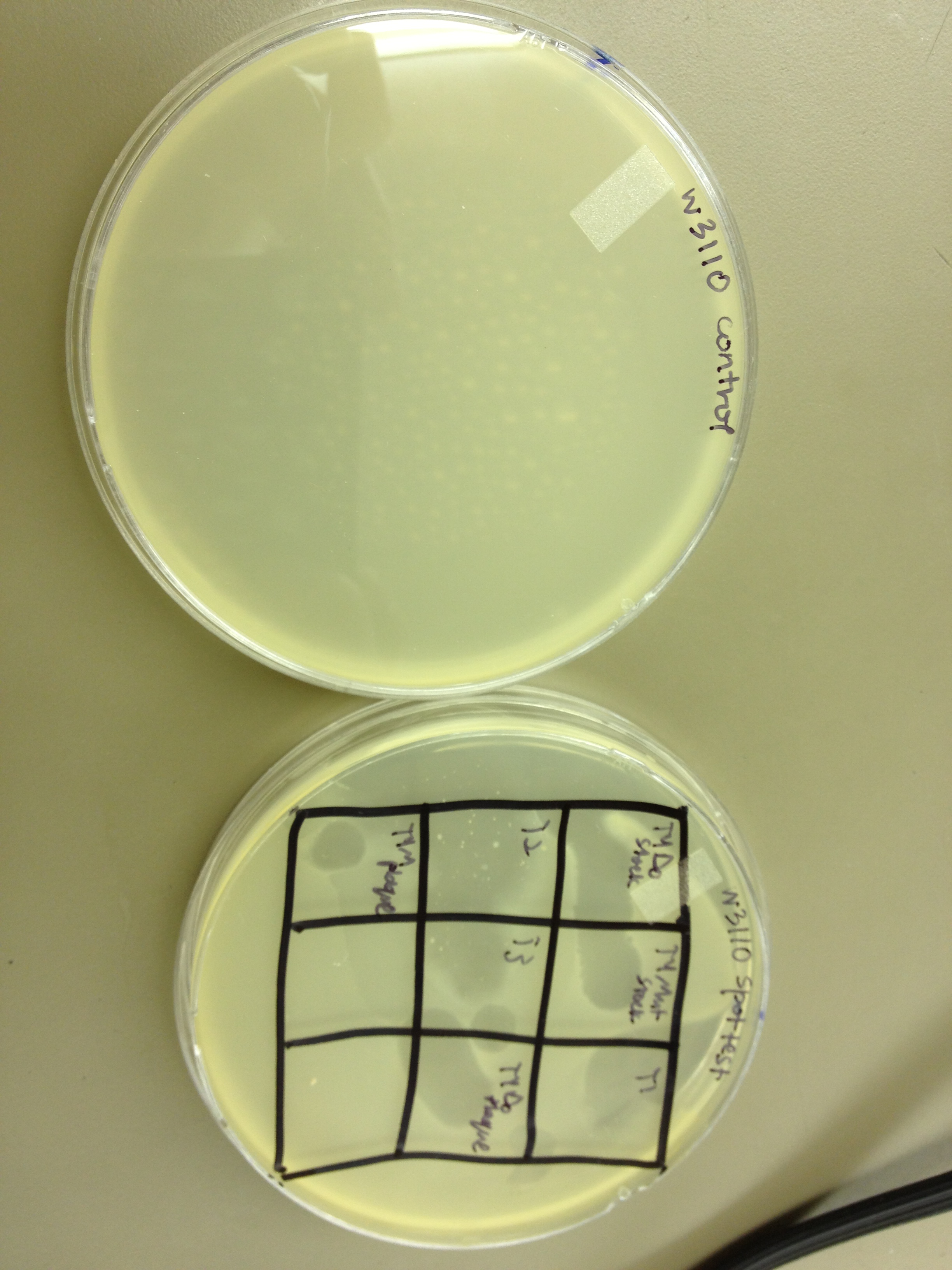
Results from 5/22/13 - We left the plates in the incubator for 48 hours, which caused contamination on many plates to grow.
When checked at 24 hours, the T4 mutagenized stock had a web plate at 10^-3 and less than 5 plaques at 10^-6. This experiment will need to be redone from 10^0 down through 10^-6. The whole mutagenesis may need to be redone if this only represents a dilution of our titer when we were trying to grow it in liquid culture. (We infected with ___ mL of phage at ___ titer in ____ vol of resuspended bacteria.)
Under UV light, the T4Do stock (diluted to 10^-6) has 19 plaques after being irradiated for six minutes (down from almost cleared at 0 min). Under UV light, the 180 sec UV plate spot (diluted in 1 mL) has a few hundred plaques on it, but the amount dropped significantly at 4 min 30 sec from when it was UV-ed for 0 min.
We will re-titer the T4-Mut stock so we can learn whether it was diluted or whether an infection worked. We will also re-run the UV test comparing the T4-Mut (10^-3) with the T4-Do stock (at 10^-6) for survivability. The mutagenized phage should survive better.
Since the loss of plaques seemed to level off for the T4Do stock at 5:15 (23 plaques) and 6 min (19 plaques), we will test a 7 min and 8 min timepoint to see if it stays level, suggesting these phage have multiple genomes and are severely mutated.
5/24/13
5/24/13
5/27/13
5/29/13
5/31/13
I took out the plates from last times experiments.
The titer for T4 was unsuccessful on the E.Coli B, so we'll need to try that again.
Also, today Jade taught me how to set up the website- so I worked on that.
 "
"