Team:NCTU Formosa/project
From 2013.igem.org
(→Small RNA) |
Darren820823 (Talk | contribs) (→Mechanism) |
||
| Line 49: | Line 49: | ||
====Mechanism==== | ====Mechanism==== | ||
| - | <p>We want to create a light sensor in Escherichia coli, but Photoreceptors are not found in E.coli. Therefore, we introduce a "two component system" (Cph8) including a photoreceptor (Cph1) that is | + | <p>We want to create a light sensor in Escherichia coli, but Photoreceptors are not found in E.coli. Therefore, we introduce a "two component system" (Cph8) including a photoreceptor (Cph1) that is from Synechocystis .</p> |
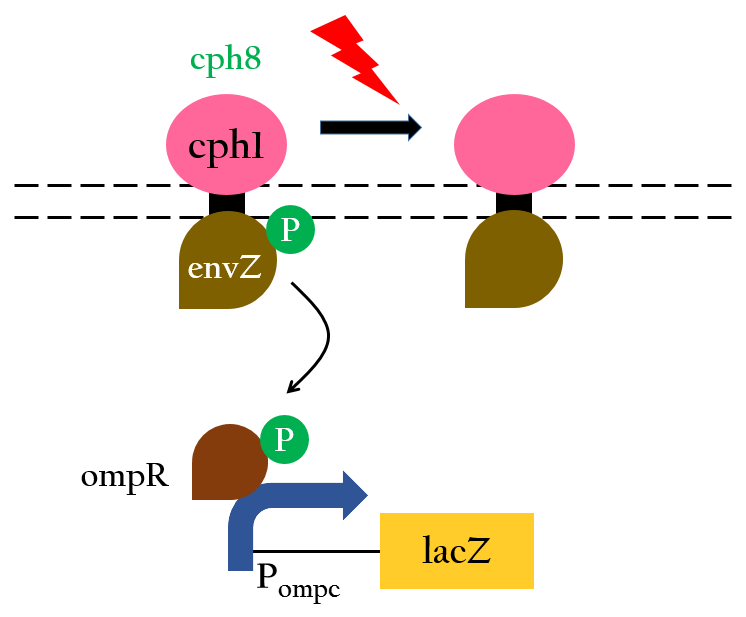
<p>The chimera Cph8 is consist of the phytochrome Cph1 and the histidine kinase domain and response-regulator from EnvZ-OmpR. Cph8 autophosphorlates ompR, then phosphorylated-ompR activates the P<sub>ompc</sub> and turns on the downstream gene</p> | <p>The chimera Cph8 is consist of the phytochrome Cph1 and the histidine kinase domain and response-regulator from EnvZ-OmpR. Cph8 autophosphorlates ompR, then phosphorylated-ompR activates the P<sub>ompc</sub> and turns on the downstream gene</p> | ||
Revision as of 12:30, 25 September 2013
A multiple regulated-system was built using three different regulation mechanisms including red light, temperature, and sRNA. In other words, it is multitasking genetic engineered machine that can express a variable genes depending on the different command given.
Contents |
Introduction
The majority of the previous iGEM projects focused on expressing certain genes to achieve a specific task such as sensing a certain phenomenon. This year, we took a step forward in exploring the potential of E. Coli, thus building a system capable of regulating multiple genes. The E.colightuner is built by three regulated-systems, light control system, temperature regulated-system and sRNA regulated-system. We used noninvasive factors such as red light and temperature to create different conditions under which E. coli can express different genes. We also introduce a new sRNA regulated-system that haven't been widely applied in iGEM. This sRNA regulated-system acts as a specific ribosome binding site (RBS) and can be shut down by nucleotide base pairing on the mRNA level. This way, we could turn off the unnecessary reaction in a multiple regulating system.
The E.colightuner composed by a meticulous design of biobricks. Through the gene function of inhibiting and inducing, the E.colightuner acts as a lock. It means that by changing environmental conditions of our E. Coli, we can switch the expression of E. Coli to the desired one. To put it simply, in contrast that only a single task can be managed genetically by previous projects, this system is a genetic-powered machine that can do multitasking.
The E.colightuner is believed to use in a pathway which required a multiple control. In our goal, we apply four plant growth hormones to the E.colightuner. By controlling the reacting conditions, the E.colightuner produces the specific hormone we needed for plants growing. We design a closed incubator with bulb for changing culture environment. There is a filter in the incubator preventing the bacteria coming out when extracting the hormone. We hope this idea could apply on the callus culture. (a more powerful ending?)
Light-regulated system
Introduction
Light, causing the change of day and night, is a common energy of nature. Light is indispensable for many organisms such as plants and photosynthetic bacteria to growth. For the purpose of sensing the light, bacteria use either photocaged molecules and enzymes or photoreceptors with a light-sensitive domain harboring a small light-absorbing ligand called chromophore. And the chromophore could absorb light in a defined wavelength in order to initiate a lighttriggered reaction. These light-mediated control of gene expressions such as protein function and metabolic process in living microbes is now a developing field of research. For instance, light sensor is a popular researching field in iGEM.
In E.colightuner, we use the light sensor as a switch when turning our pathway on or shutting down. Light provides an independent control with a noninvasive and spatiotemporal fashion. Different from common promoters, the light promoter senses the specific wave light directly. That means we don't have to add extra substrates to induce the promoter. This way, we could achieve a pathway regulation system without changing the reacting environment like adding extra induction substrates .
Mechanism
We want to create a light sensor in Escherichia coli, but Photoreceptors are not found in E.coli. Therefore, we introduce a "two component system" (Cph8) including a photoreceptor (Cph1) that is from Synechocystis .
The chimera Cph8 is consist of the phytochrome Cph1 and the histidine kinase domain and response-regulator from EnvZ-OmpR. Cph8 autophosphorlates ompR, then phosphorylated-ompR activates the Pompc and turns on the downstream gene
The phytochrome Cph1 from the cyanobacterium Synechocystis sp. PCC6803 is the first member of the plant photoreceptor family that has been identified in bacteria. It is a dimeric receptor protein that binds phycocyanobilin (PCB) as a red-lightabsorbing chromophore.4,5
EnvZ, a dimeric osmosensor, is a multidomain transmembrane protein and one of the best characterized two component histidine kinases from E. coli.Besides the periplasmic receptor domain that is flanked by two transmembrane helices, it possesses a C-terminal 228-residue histidine kinase domain that is located in the cytoplasm6. Upon changes of extracellular osmolarity, EnvZ specifically phosphorylates its cognate response regulator OmpR, which, in turn, regulates the Pompc 7.
The fusion protein Cph8 was generated to combine the light-sensing function of Cph1 with the output function of EnvZ.
Red light inhibits the autophosphorylation of Cph8 and turns off the gene expression. Figure 3 depicts this mechanism.8 In this case,lacZ is the reporter gene.
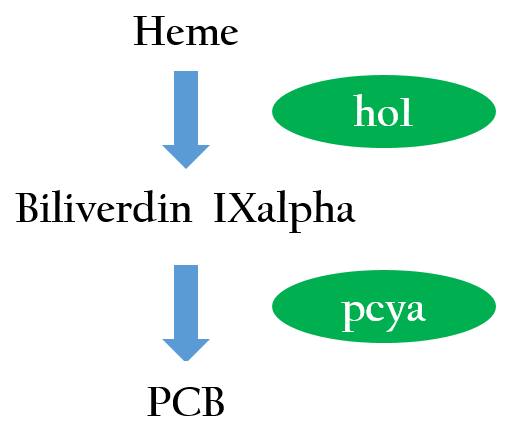
The functional expression of a phytochrome domain in E. coli requires the biosynthesis of the respective bilin chromophore PCB. Two phycocyanobilin(PCB)-biosynthesis genes (ho11 and pcyA2) transplanted from Synechocystis to E.coli encoding a heme oxygenase and bilin reductase, respectively, is essential to convert heme into PCB. Figure 2 depicts the phycocyanobilin-biosynthesis pathway.3
圖 Cph8的"C"是大寫
Design of Red promoter
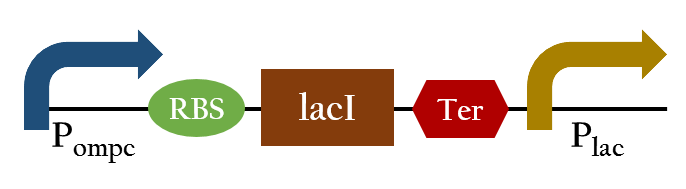
Because the Pompc turns off when exposing to red light, we want to construct a promoter which can detect red light and turn on downstream genes. Therefore, we add the lacI and the Plac behind Pompc. In normal condition, the Pompc would turn on and the lacI expresses, then the product of lacI represses the Plac. In contrast, when exposing to red light, the Pompc would be repressed and the Plac turns on. Finally, we created a new promoter that can turn on under red light.
Exposing to red light, the phosphorylation activity is hindered so the promoter remains repressed and the downstream genes cannot be translated. Such negative control, however, is more complicated and less straightforward than a simple positive control. Therefore , we converted negative control of Pompc into positive control by building a biobrick called Pred.
In the presence of red light, Pompc is shut down and lacI cannot be expressed to repress Plac. As a result, Plac is activated and all the downstream genes can be expressed. In the absence of red light, Pompc is activated as phosphorylation is triggered and lacI is expressed. Consequently, lacI respressed Plac and the genes downstream cannot be expressed. By using Pred, we can achieve positive control using red light.
Reference
- part BBa_I15008;MIT Registry of Standard Biological Parts
- part BBa_I15009;MIT Registry of Standard Biological Parts
- Levskaya, A. et al .(2005). Engineering Escherichia coli to see light. Nature, 438(7067), 442.
- Kehoe DM, Grossman AR (1996) Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 273(5280):1409–1412
- Yeh KC, Wu SH, Murphy JT, Lagarias JC (1997) A cyanobacterial phytochrome two-component light sensory system. cience 277 (5331):1505–1508
- Dutta R, Qin L, Inouye M (1999) Histidine kinases: diversity of domain organization. Mol Microbiol 34(4):633–640
- Forst SA, Roberts DL (1994) Signal transduction by the EnvZ–OmpR phosphotransfer system in bacteria. Res Microbiol 45(5–6):363–373
- Thomas Drepper, Ulrich Krauss,Sonja Meyer zu Berstenhorst, Jörg Pietruszka, Karl-Erich Jaeger.(2011).Lights on and action! Controlling microbial gene expression by light. Appl Microbiol Biotechnol, 90:23–40 DOI:10.1007/s00253-011-3141-6
Temperature-regulated system
Introduction
Cells are constantly subjected to changing environmental conditions and one example of such a changing environmental condition is the temperature. Comparing with the chemical substance, temperature is more convenient and lower costing. Moreover, temperature is also less burden to the organisms. Therefore, there are a few organisms use temperature as a regulate factor. And there's a mechanism found in different organisms, that makes the cell respond to thermal changes, is the RNA thermometer.
An RNA thermometer is a temperature-sensitive non-coding RNA molecule which regulates gene expression. The expression of heat-shock, cold-shock and some virulence genes are coordinated in response to temperature changes.There are several systems suggested in literature that are based on RNA secondary structure. This structural transition can then expose or occlude important regions of RNA such as a ribosome binding site, which then affects the translation rate of a nearby protein-coding gene, so we can say that the RNA thermometers are just like riboswitches.
Apart from protein-mediated transcriptional control mechanisms, translational control by RNA thermometers is also a widely used regulatory strategy.
Mechanism
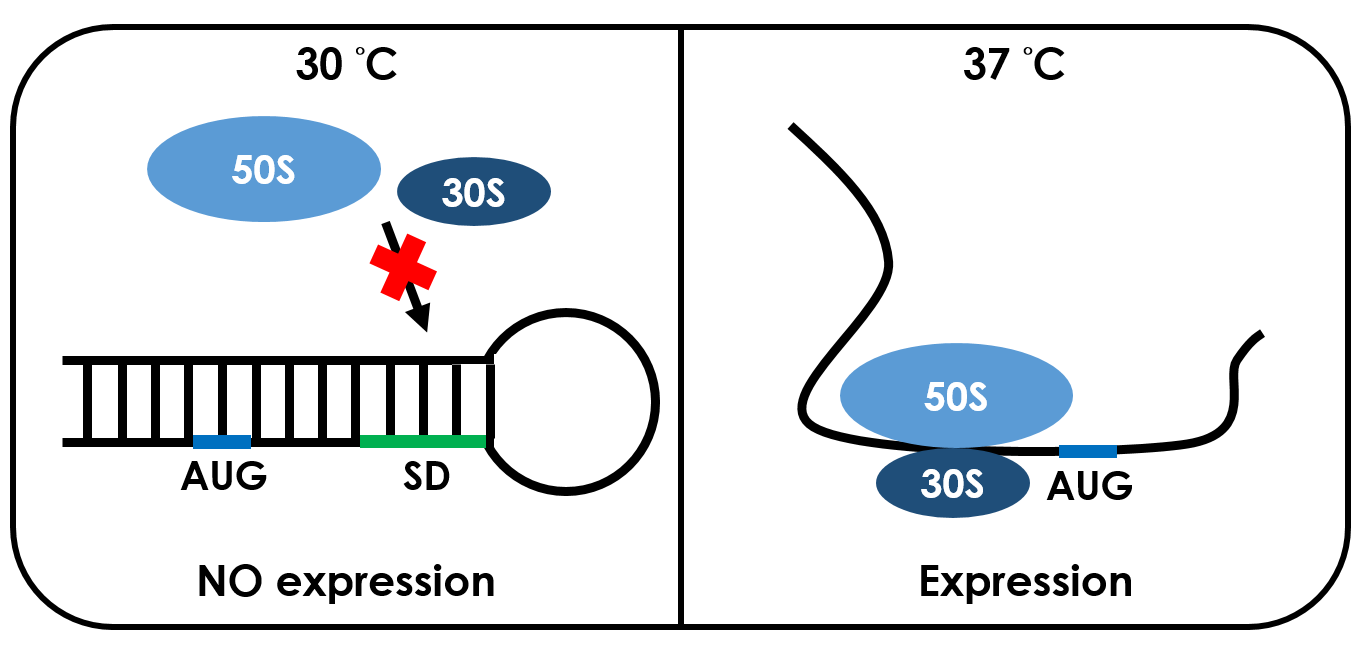
RNA thermometers are found in the 5' UTR of messenger RNA, upstream of a protein-coding gene. They are able to occlude the ribosome binding site (RBS) and prevent translation of mRNA into protein. Therefore, we use temperature to unlock this "RBS lock".
Unlike normal RBS, the temperature RBS(37°CRBS) has a unique rather stable hairpin structure which shape like a lock. If the temperature drops below a certain temperature, the base-pairing neucleotides on the RNA will form a stable hairpin structure, occluding the Shine-Dalgarno sequence thus disabling the ribosome to bind. The base-pairing of this RNA region will block the expression of the protein encoded within. Since the structure is sustained by base pairing, heat can be employed to break the hydrogen bonds. So when it reaches 37 degree celcius, the bonds will be broken, the hairpin would be unfolded, causing the Shine-Dalgarno sequence to be exposed and permitting the binding of the ribosome, which then starts the translational machinery. This means that by raising temperature to a certain threshold, the 37°C RBS can function as a normal RBS. In this way, gene expression can be regulated on the RNA level by temperature.
這張figure可以換成之前的那張
Design
An RNA thermometer in the 5'-UTR of the Salmonella agsA gene, which codes for a small heat shock protein that can be used for temperature sensitive post-transcriptional regulation, initiates translation at 37°C. Transcription of the agsA gene is controlled by the alternative sigma factor s32. Additional translational control depends on a stretch of four uridines that pair with the SD sequence, so they are named "FourU".They repress translation of this protein by base-pairing the Shine-Dalgardo sequence of the gene's mRNA. This prevents ribosomes from binding the gene's start codon.
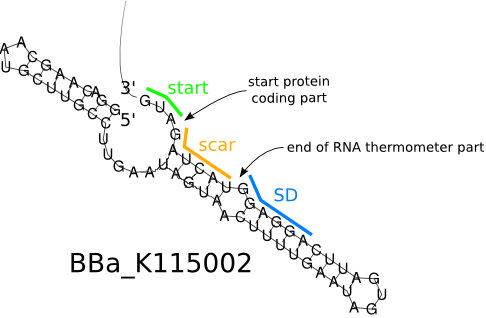
We used the RNA thermometer to build up a low-temperature released system to control the expression of targeted protein. A specific ribosome binding site (RBS) BBa_K115002 with high translation activity at high temperature(> 37°C) and low translation activity at room temperature was used to design the temperature-dependent genetic circuit in E. coli, with a green fluorescent protein (GFP) used as the reporter protein.
In our project, we use this 37°C RBS in both red light induced circuit and dark response circuit and it is related to the production of GFP and BFP. We use this 37°C RBS to design our project because we want to use different conditions to produce different substances. Therefore, the characteristic and sensitivity of 37°C RBS is just fit in our purpose which we want to multiple regulate our system through temperature.
Small RNA-regulated system
Introduction
Base pairing offers a powerful way for one RNA to control the activity of another. Both prokaryotes and eukaryotes have many cases which a single-stranded RNA base pairs with a complementary region of an mRNA, and as a result it prevents expression of the mRNA.
RNA interference(RNAi), also called post transcriptional gene silencing is a process in which RNA molecules inhibit gene expression by destroying specific mRNA molecules. In 2006, Andrew Fire and Craig C.Mello shared the Nobel Prize in Physiology or Medicine because of their study on RNA interference9.This powerful gene silencing tool in eukaryotes has been used in many research. As their counterparts in bacteria, small non-coding RNAs(sRNAs) are important regulatory roles.
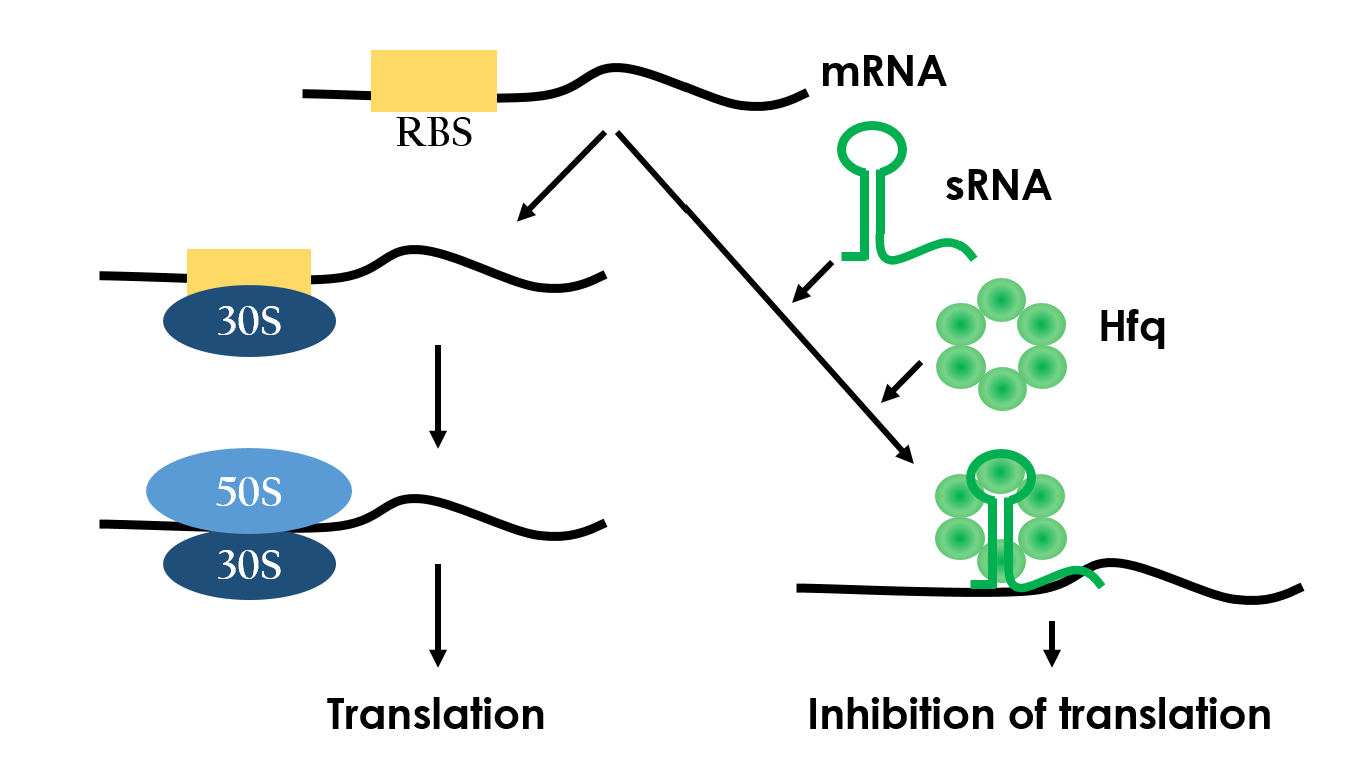
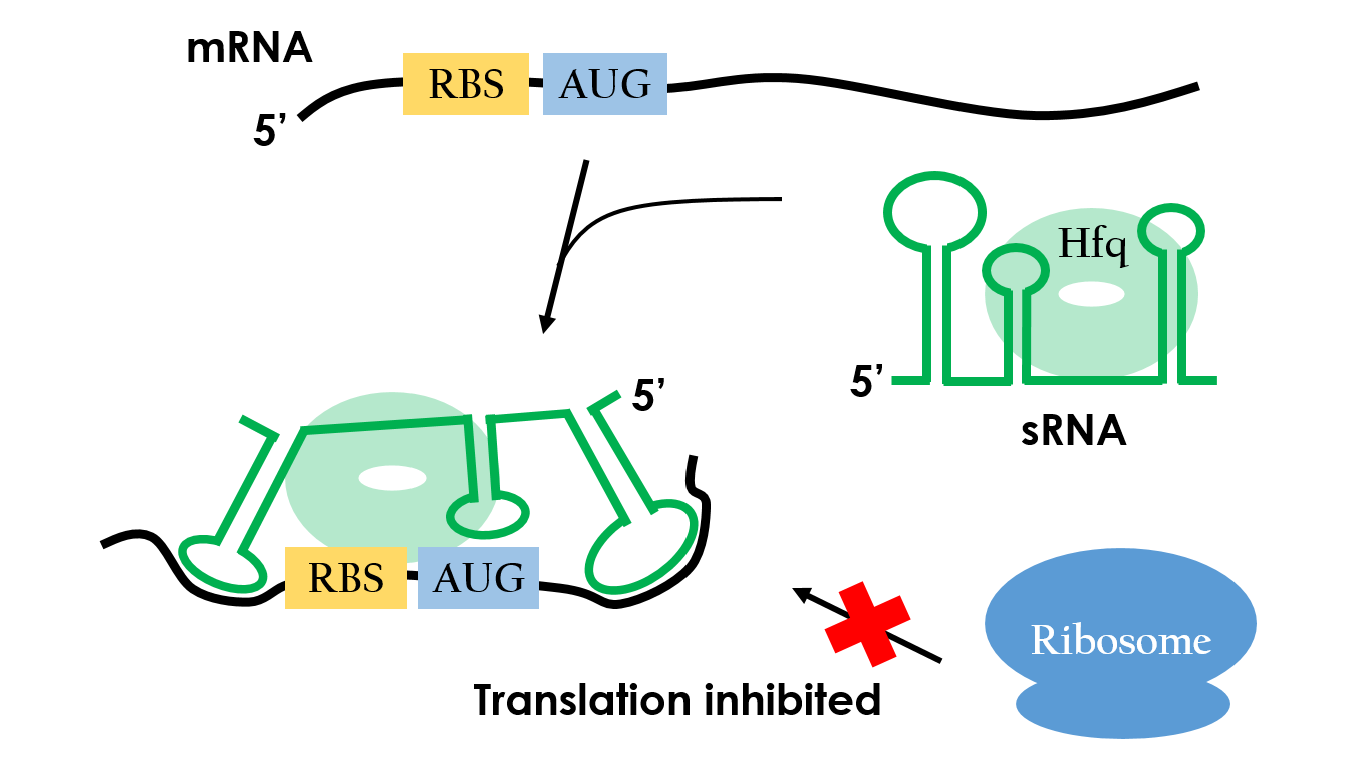
Small RNAs (sRNAs) have become increasingly significant in playing the role of bacterial gene regulation. Most sRNAs interact with the targeted mRNAs by imperfect base pairing, reducing the translation efficiency and recruiting chaperones such as Hfq for translation termination. The sRNAs would bind on the target with some of it's own sequences wind to create hairpins-like structure, and the chaperons would stuck between the hairpins in order to protect the sRNA-mRNA combination from degrading. In the end, sRNAs regulate gene expression by forestalling translation. It is an effective and specific regulated-system that functions under RNA level. Since this regulated-system has already been employed in vivo, it is necessarily to design artificial sRNA that targets specifically to the desired genes. This way, we can prevent the sRNA from effecting undesired genes.
Unlike the regulated-system that popular used in iGEM projects(e.g., Ptet & tetR, Plux & LuxR, etc.), sRNA regulated-system seems more efficient. Because the inhibition works under RNA level, which means the energy waste in E.coli is less than other system(producing proteins to regulate).
Figure 8 depeicts how the small RNA chaperone, Hfq, associates with the small RNA and represses a target mRNA.10
Mechanism
Oxidative stress in E.coli provides an example of a sRNA-regulated system.Exposing to reactive oxygen species, bacteria would induce antioxidant defense genes. Hydrogen peroxide activates the transcription activaor OxyR, which controls the expression of several inducible genes. oxyS, one of these genes, codes for a samll RNA.
The oxyS RNA is a short sequence(109 nucleotides) which doesn't code for protein. It has more than 10 target mRNAs. Fig 9 shows the mechanism of repression of one target, the flhA mRNA. The oxyS RNA has three stem-loop double stranded RNA structures, and the loop closest to the 3’ terminus is complementary to a sequence preceding the initiation codon of flhA mRNA. Base pairing between oxyS RNA and flhA RNA prevents the ribosome from binding to the initiation condon, so the translation would be repressed.11,12
figure將flha與Oxys拿掉
Design
(text)需要更詳盡的設計流程
The sRNA employed in this project was picked from a library of artificial sRNA that was constructed by fusing a randomized antisense domain of Spot42, the scaffold that is known to recruit the RNA chaperons. The sRNA we picked contains a consensus sequence, 5’-CCCUC-3’, that can base pair with the SD sequence due to complementarity. This sRNA, as expected, effectively regulates gene expression by reducing translation efficiency and recruiting Hfq. In addition, this sRNA shows high specificity against its targeted gene, ompF, as it doesn’t hold significant activity against other genes from E. Coli genome (Sharma and others, 2011).
The sRNA picked is competent, but we hoped that it can target any desired gene. With that said, we designed a RBS by employing the sRNA targeting region from ompF and making the AUG codon sufficiently apart from the SD sequence for ribosome binding. By adding this RBS to the upstream of any desired gene, the gene can be regulated by sRNA.
Reference
- Xu, S.; Montgomery, M.; Kostas, S.; Driver, S.; Mello, C. (1998). "Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans". Nature 391 (6669): 806–811 DOI:10.1038/35888
- Jörg Vogel , Ben F. Luisi.(2011). Hfq and its constellation of RNA. Nature Reviews Microbiology, 9:578-589
- E.K. Jocelyn, S.G. Elliott , T.K. Stephen, "Lewin's Genes X.-10th ed.", Jones & Bartlett, Sudbury, MA, 2011.
- Karen M. Wassarman.(2002). Small RNAs in Bacteria: Diverse Minireview Regulators of Gene Expression in Response to Environmental Changes Changes. Cell, 109:141–144
Mechanism of E.colightuner
Introduction
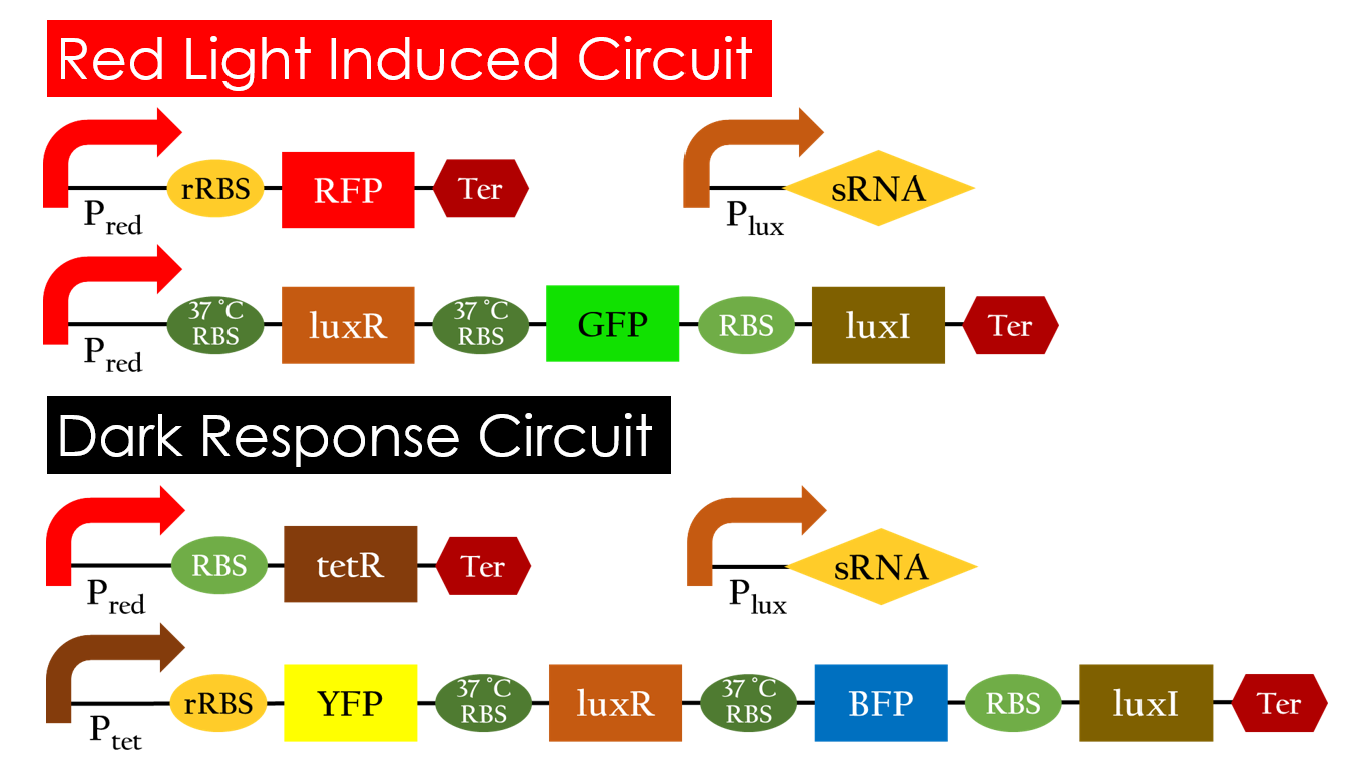
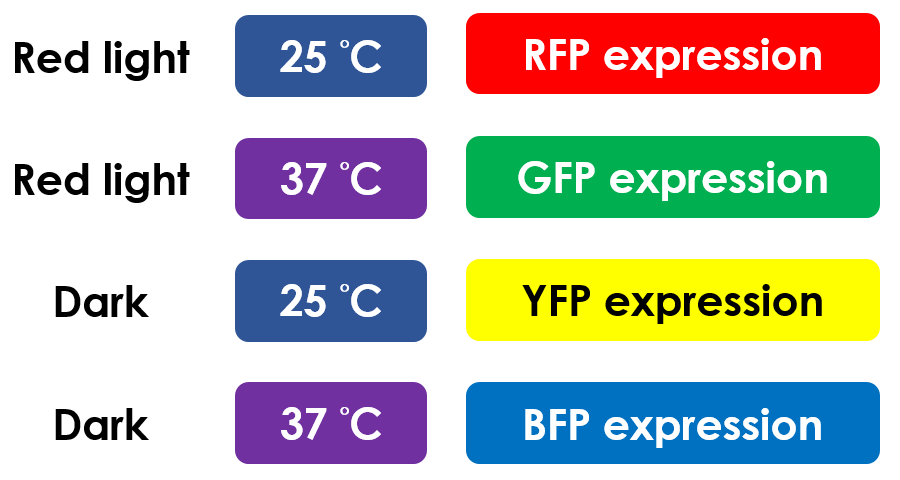
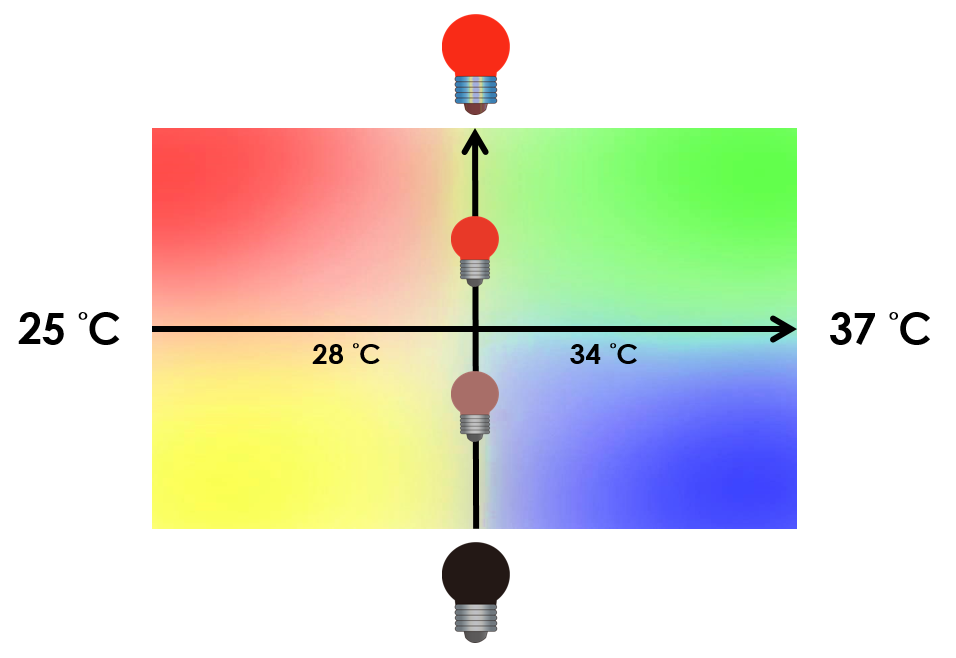
This is the pathway we designed by using the three different regulating factors. We tend to set up four conditions for the E.coli to work by paring the light and the temperature controls. Distinguished by whether red light exists or not, the pathway divided into two parts, the Red Light Induced Circuit and the Dark Response Circuit. We use four different fluorescent proteins as reporter genes to test that whether the switch of our pathway is successful.
Red Light Induced Circuit
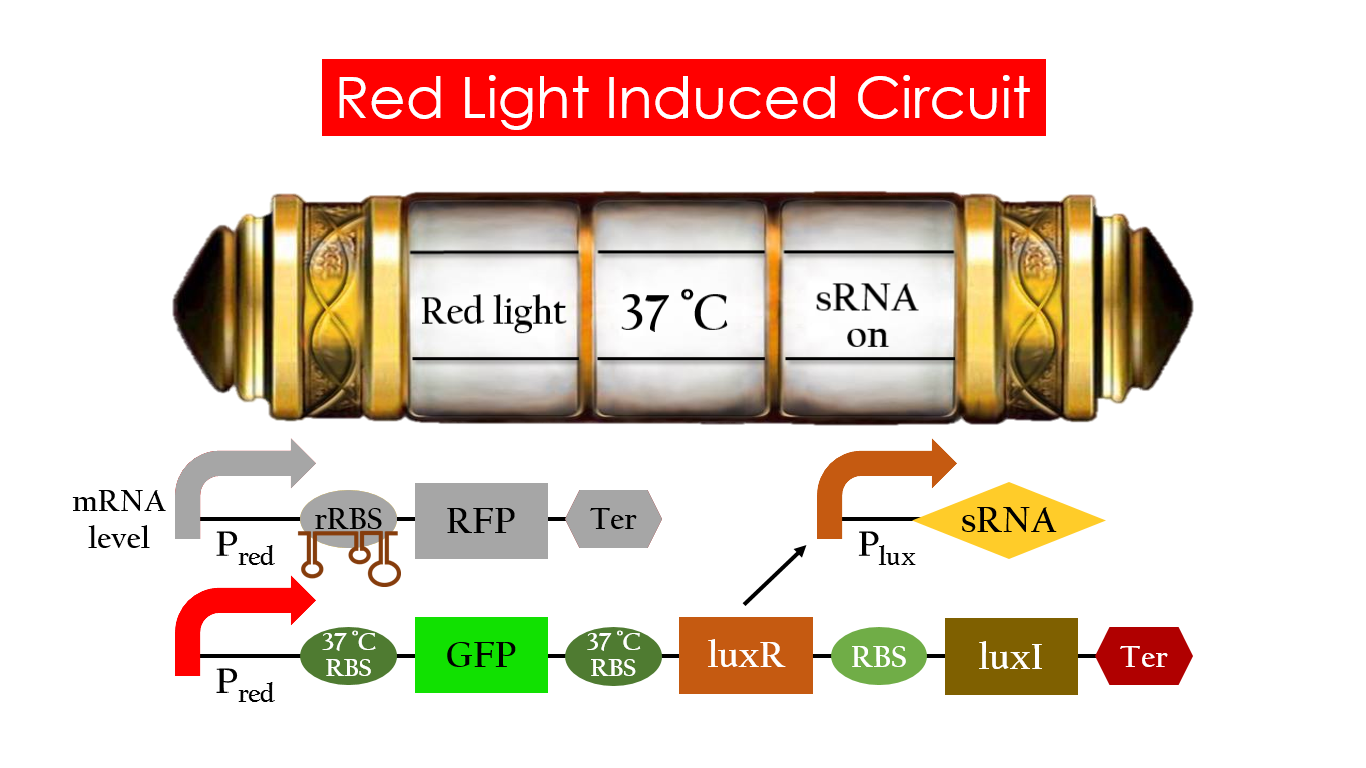
At 30°C and under red light, Pred is activated for translation proceeds. However, ribosomes can only bind to the normal RBS, as 37°C RBS (tRBS) forms a hairpin structure, forestalling ribosomes from binding. The only gene downstream of the normal RBS is RFP, so only RFP would be expressed.
On the other hand, with red light, 37°C RBS unfolds at 37°C , resulting in the expression of both LuxR and GFP. LuxR would binds with AHL to form a complex that activates Plux. The activation of Plux produces sRNA that binds to the normal RBS on mRNA level, blocking it from ribosomes. As a result, RFP would not be expressed and only GFP remains at 37°C.
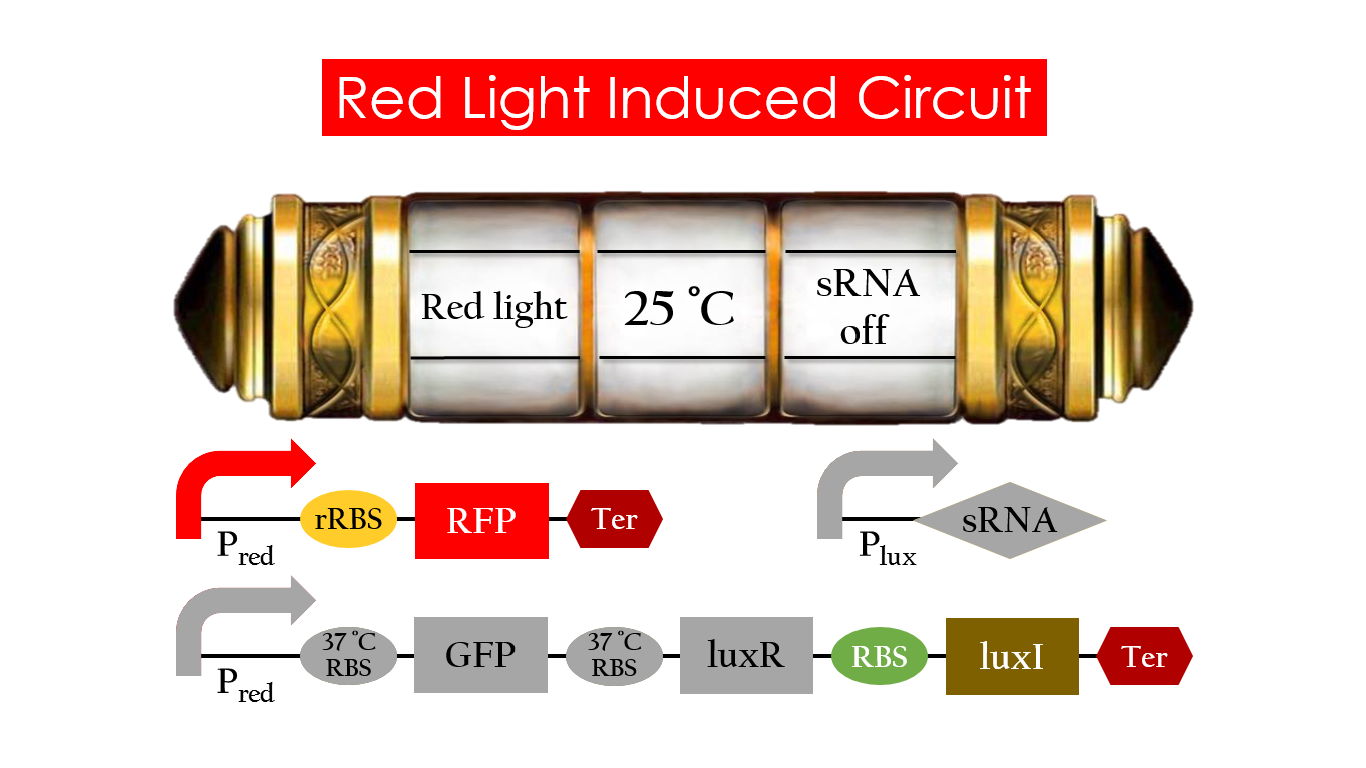
Without red light, Pred would not be activated, and therefore, the red light induced circuit is completely shut down in the dark.
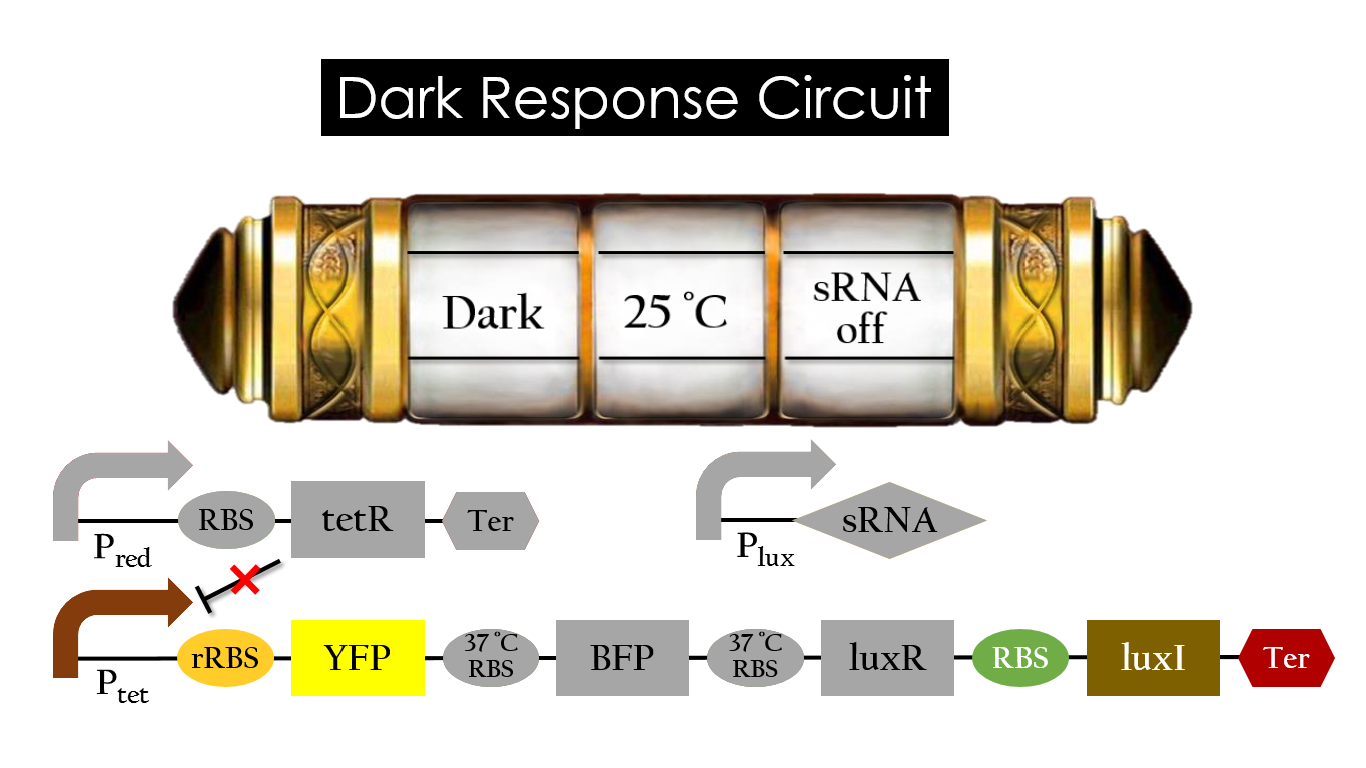
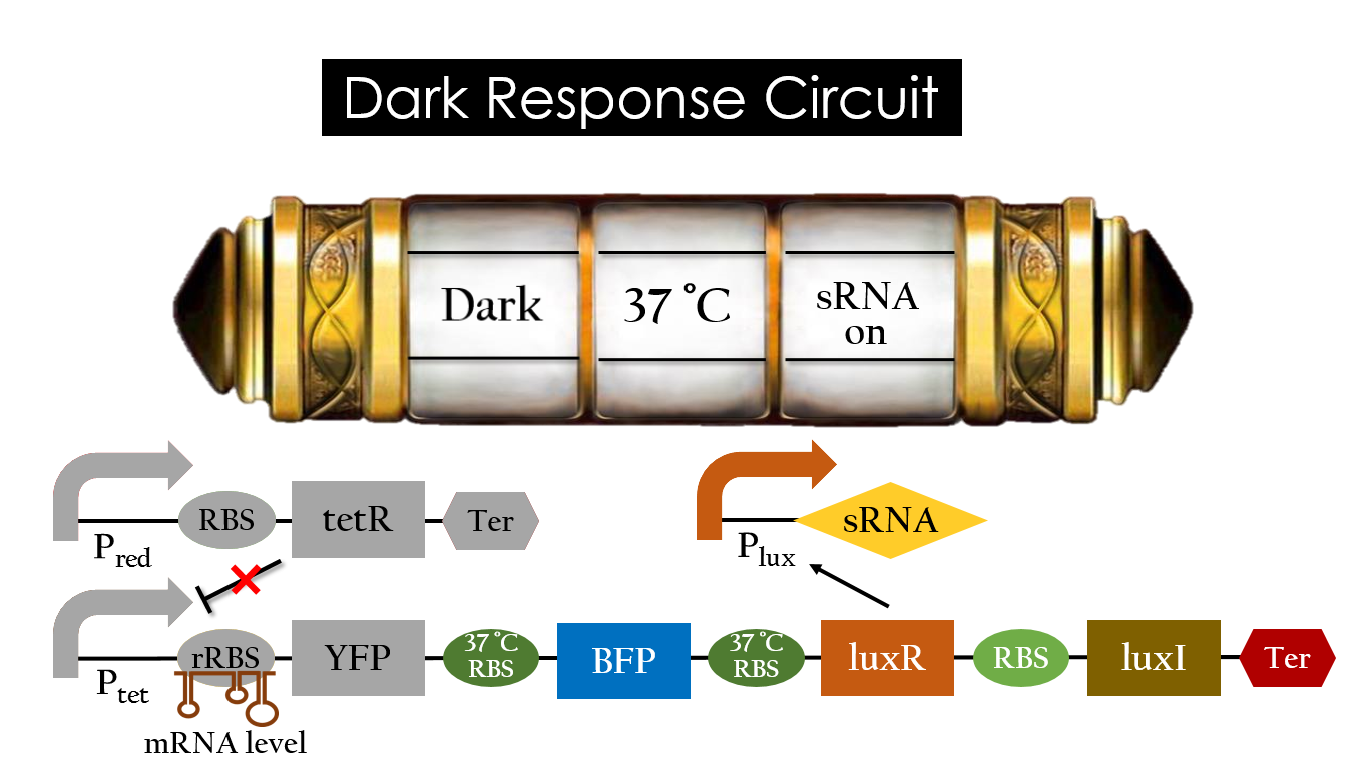
Dark Response Circuit
Pred activates in the presence of red light, producing tetR that represses Ptet. In other words, the dark induced part is inactive in the exposure to red light and active without red light. At 30°C in the dark, only normal RBS functions to express YFP while 37°C RBS remains as a hairpin structure.
At 37°C, however, 37°C RBS unfolds to express LuxR and BFP. Just like the mechanism employed in the light induced part, LuxR forms a complex with AHL to activate Plux that produces the sRNA to block normal RBS. This way, the YFP downstream of YFP cannot be expressed and BFP is expressed at 37°C.
Summary
This is the pathway summary of the E.colightuner. There is one kind of fluorescent protein in each condition we set. It means that we can control four gene expressions by using the E.colightuner. The only thing we have to do is changing the different environmental conditions for specifically expression.
Future Work
The multiple regulated-system we have created in this project consisted of two different parts: light induced part and dark induced part. Each part regulates two different genes, resulting in a total of four genes that can be regulated. By adding new parts to the system, it is plausible that the system can regulate more genes. We tend to do that by employing more light sensing promoters. Each light sensor we add, we would be able to regulate two more different genes by employing regulation mechanism of 37°C RBS and sRNA.
Application
Taiwan is renowned for Phalaenopsis, producing more than 35 million Phalaenopsis each year. There is no doubt that Taiwan has become "The Kingdom of Phalaenopsis". The technique we usually use to grow Phalaenopsis is plant tissue culture, thus, we intend to apply our system to plant tissue culture as well.
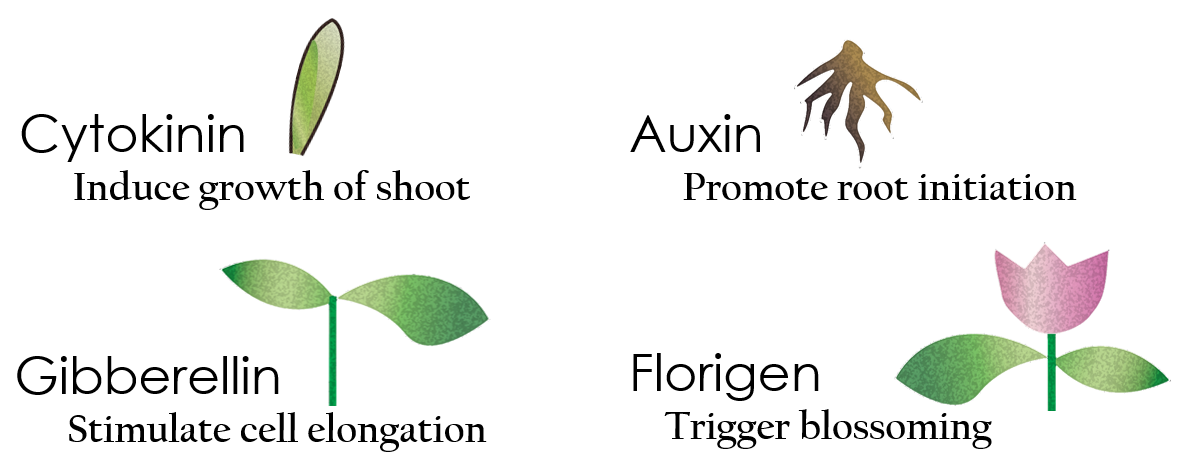
Tissue culture is the growth of tissues or cells separate from the organism. This is typically facilitated via use of a liquid, semi-solid, or solid growth medium, such as broth or agar. Moreover, hormones are added to the growth medium with a view to meeting what the plants need during growth period. However, the hormones needed during each growing state are not the same. So, we need to figure out a device which can generate multiple hormones. By adding our engineered E.coli to the growth medium, the plants can get multiple hormones secreted by E.coli under different circumstances. We chose four common hormones as our genes, Auxin, Cytokinin, Gibberellin, and Florigen.
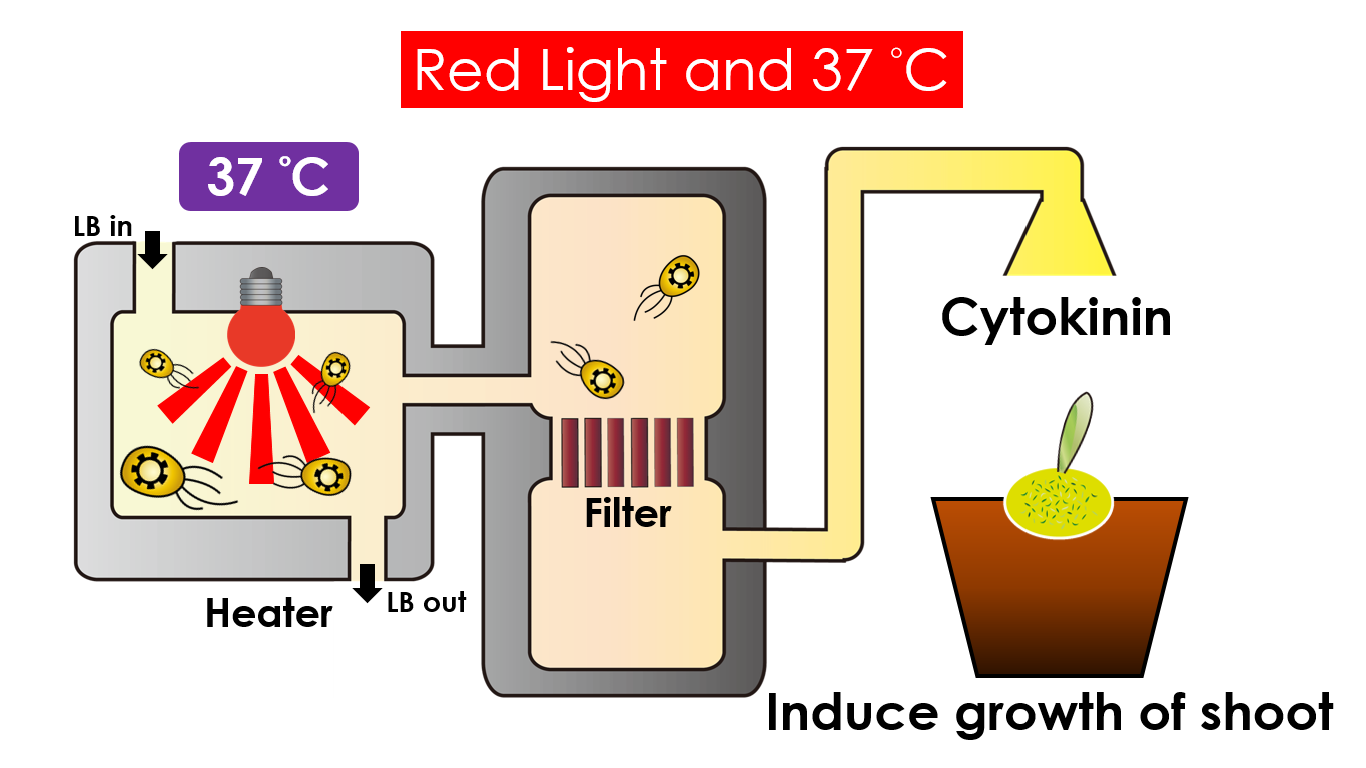
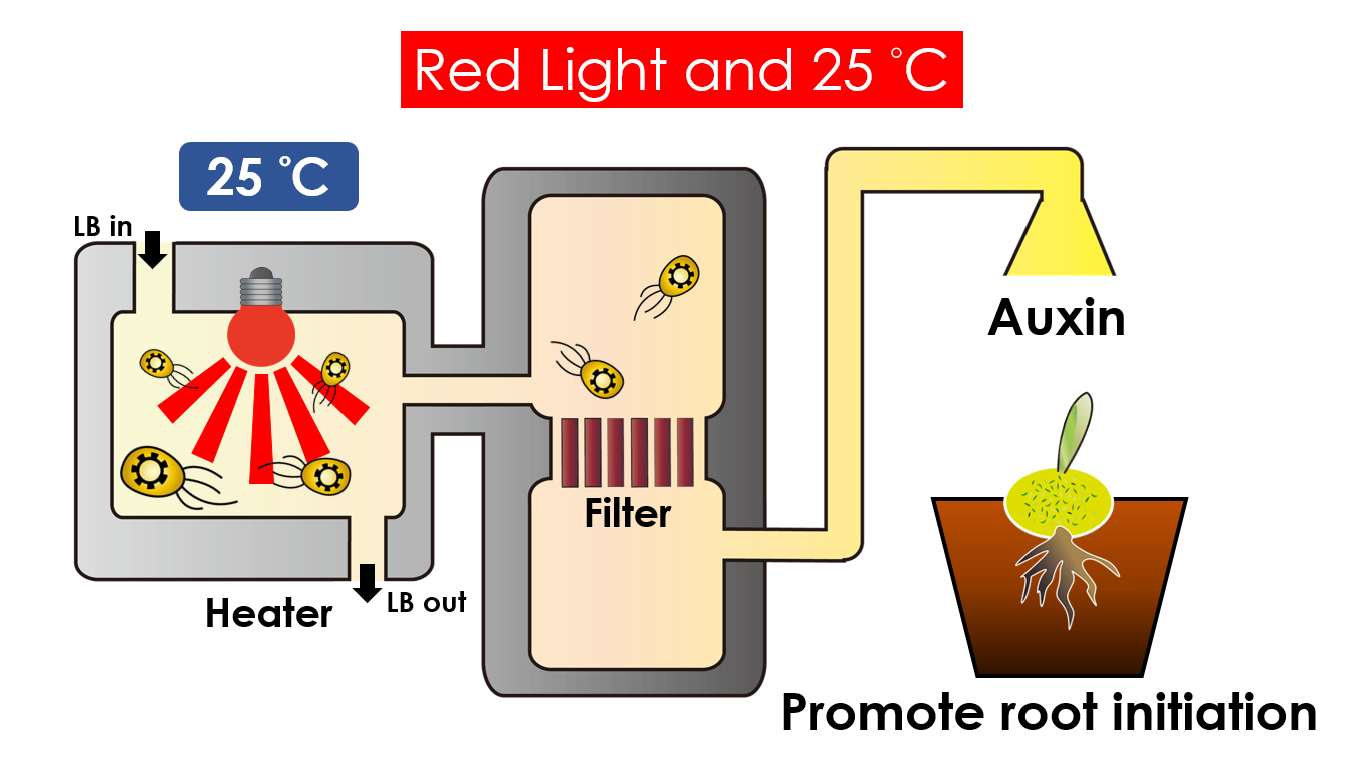
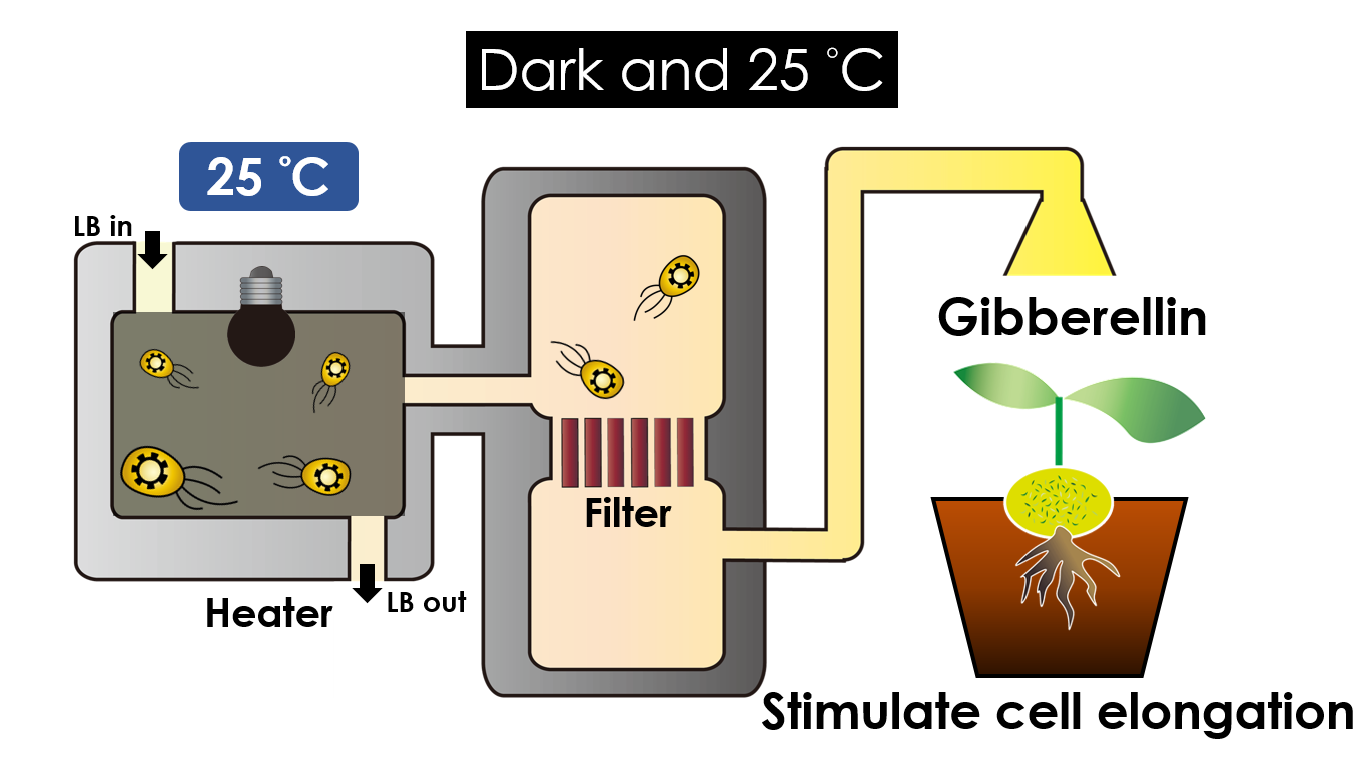
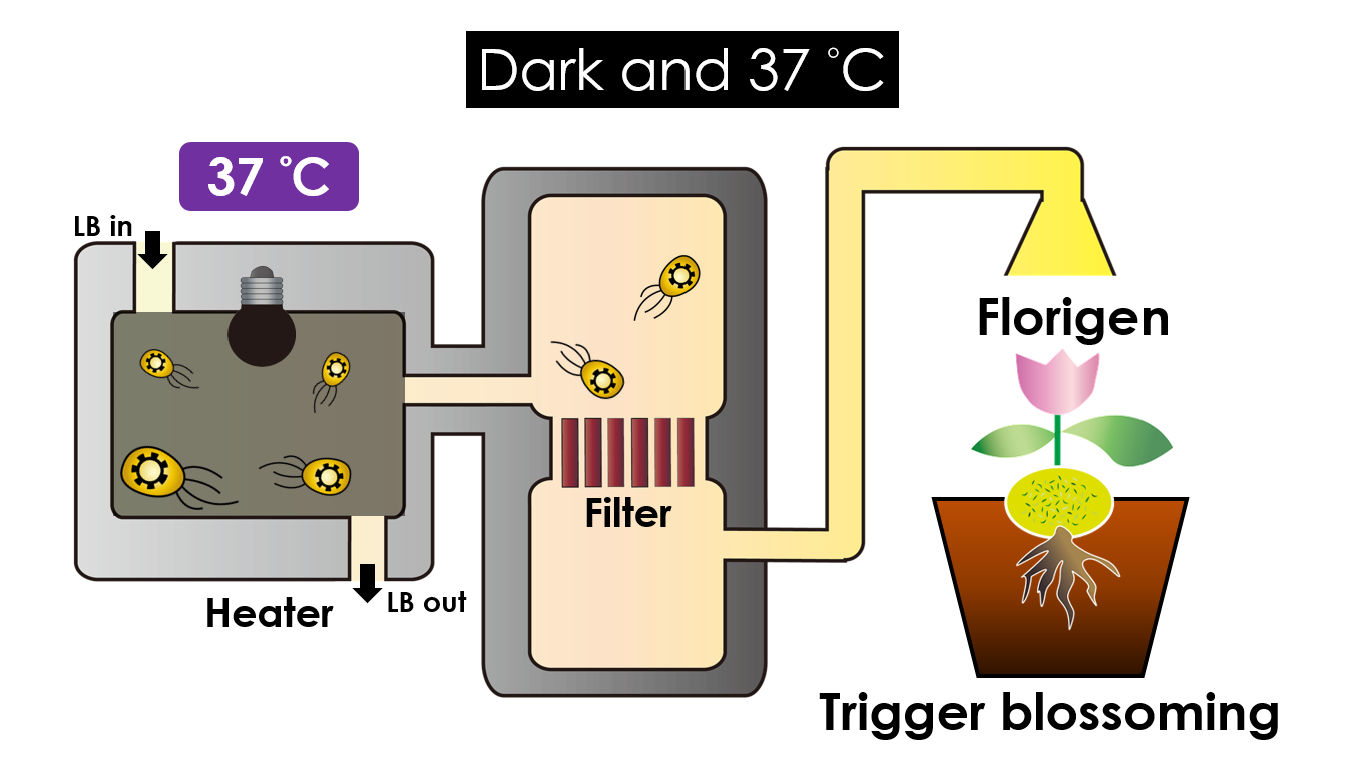
We intend to carry out our application with the device beneath. Cultured in the growth medium circulation, our engineered E.coli can absorb enough nutrition to express the desired genes properly. We also use semipermeable membrane, which allows hormones but not bacteria themselves to go through, to separate the bacteria from plant tissue. Thus, we keep the tissue culture medium sterile.
Under red light and 37°C, Cytokinin will be produced. Cytokinin is a plant growth substance which can primarily induce cell growth and differentiation in plant roots and shoots. It can decrease apical dominance which will make the main stem grow better than the other stems. In this way, all the stems are able to become stronger. Cytokinin can also signal lateral bud growth, which will make the plant bushier.
Under red light and 30°C, Auxin will be produced. Auxin can coordinate development from cellular, organs, to the whole plant. Auxin molecules in cells may cause direct responses by stimulation and inhibitions of certain gene expressions. On the cellular level, Auxin is important for cell growth. It can induce axial elongation in roots and lateral expansion which can make the roots swell. For the whole plant level, it affects the shape of the plants. Because of the different functions, each organ has uneven Auxin concentration and causes the specific shapes.
Under dark and 30°C, Gibberellin will be produced. Gibberellin is important in elongation of cells. It causes the extension of stems by inducing cell divisions and elongations. A special characteristic of it is that it can produce more mass when the plant is exposed to cold temperature.
Under dark and 37°C, Florigen will be produced. Florigen is the key hormone to trigger the plants blooming. We use a promoter that can promote transcription of FT gene,which then translate into FT proteins. FT protein will be transported via the phloem to shoot apical meristem, where it will interact with FD protein(transcription factor) to activate floral identity, thus inducing flowering.
The next big thing
On the other hand, we tend to optimize our system to achieve subtle control of the four gene expressions. We might be able to do this by taking the values between the limits we have set. Instead of 30°C and 37°C , we tend to take the values between, so we can create more conditions under which different level of expressions can be achieved. We can also vary the intensity of red light to control the level of expression. For instance, we can get defferent pigment output by tuning the temporature and varying the intensity of red light. The ultimate goal is to precisely control the level of expression of each gene.
因為Pred的直接性,在未來可以拿來測試被紅光、藍光的影響度?
Reference
- Team : Imperial_College_London -2011.igem
- Team : Kyoto -2012.igem
 "
"