Team:NCTU Formosa/results
From 2013.igem.org
| Line 48: | Line 48: | ||
<div class="li"><div class="card"> | <div class="li"><div class="card"> | ||
| - | ===Effect on E. Coli Growth=== | + | ====Effect on E. Coli Growth==== |
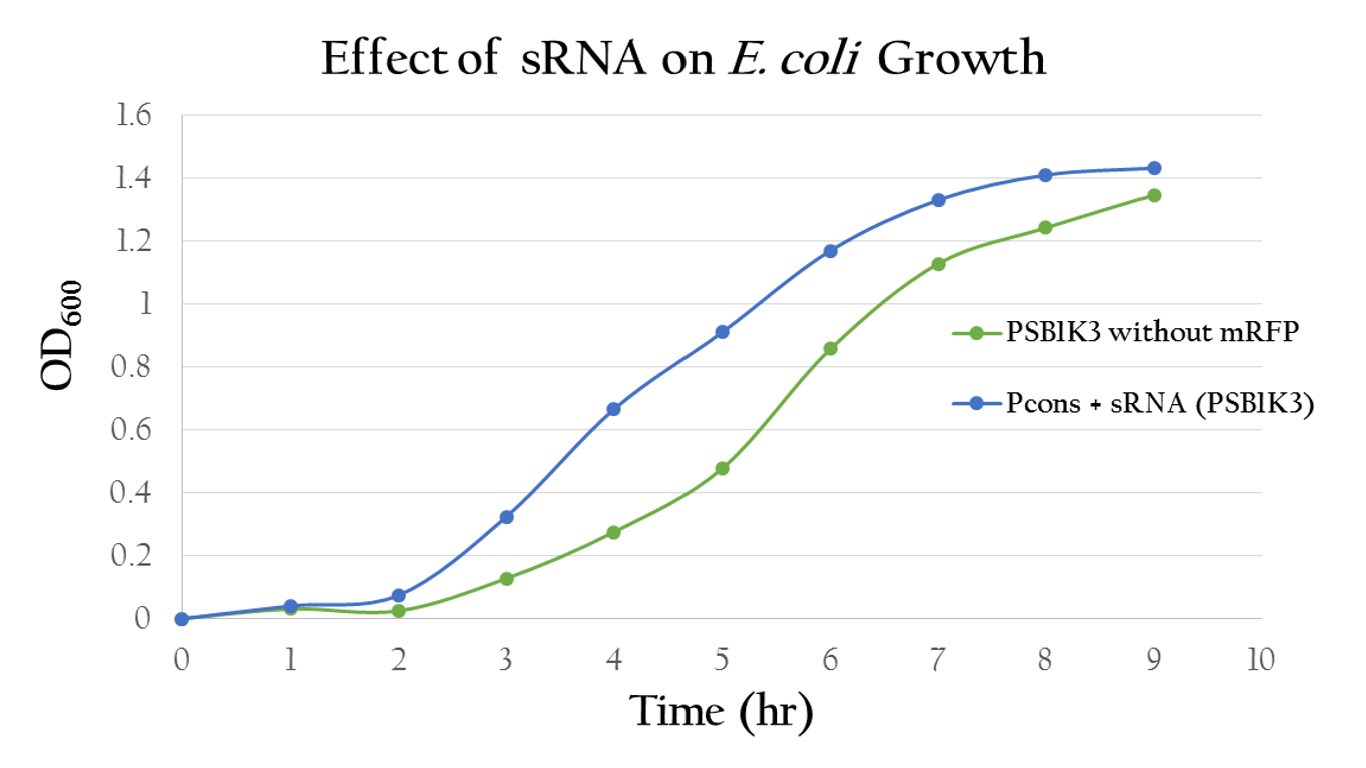
<p>To test whether or not our sRNA would effect the growth of E. Coli, we compared the growth of E. Coli with PSB1C3 (without RFP) and E. Coli with P<sub>cons</sub> + sRNA. It can be observed from ''' Figure 5''' that the growth curves of the bacteria are similar, showing no signs of growth interference. This result proves that our sRNA regulated system can be integrated into bacteria. </p> | <p>To test whether or not our sRNA would effect the growth of E. Coli, we compared the growth of E. Coli with PSB1C3 (without RFP) and E. Coli with P<sub>cons</sub> + sRNA. It can be observed from ''' Figure 5''' that the growth curves of the bacteria are similar, showing no signs of growth interference. This result proves that our sRNA regulated system can be integrated into bacteria. </p> | ||
Revision as of 15:28, 27 September 2013
The current progress of our project, including detailed information of the experimental data and the overall evaluation of the practicability of this project.
Contents |
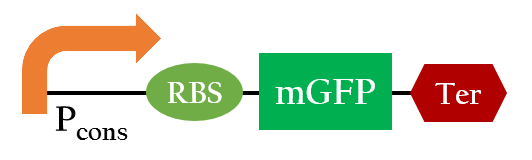
Temperature-regulated System
37°C RBS
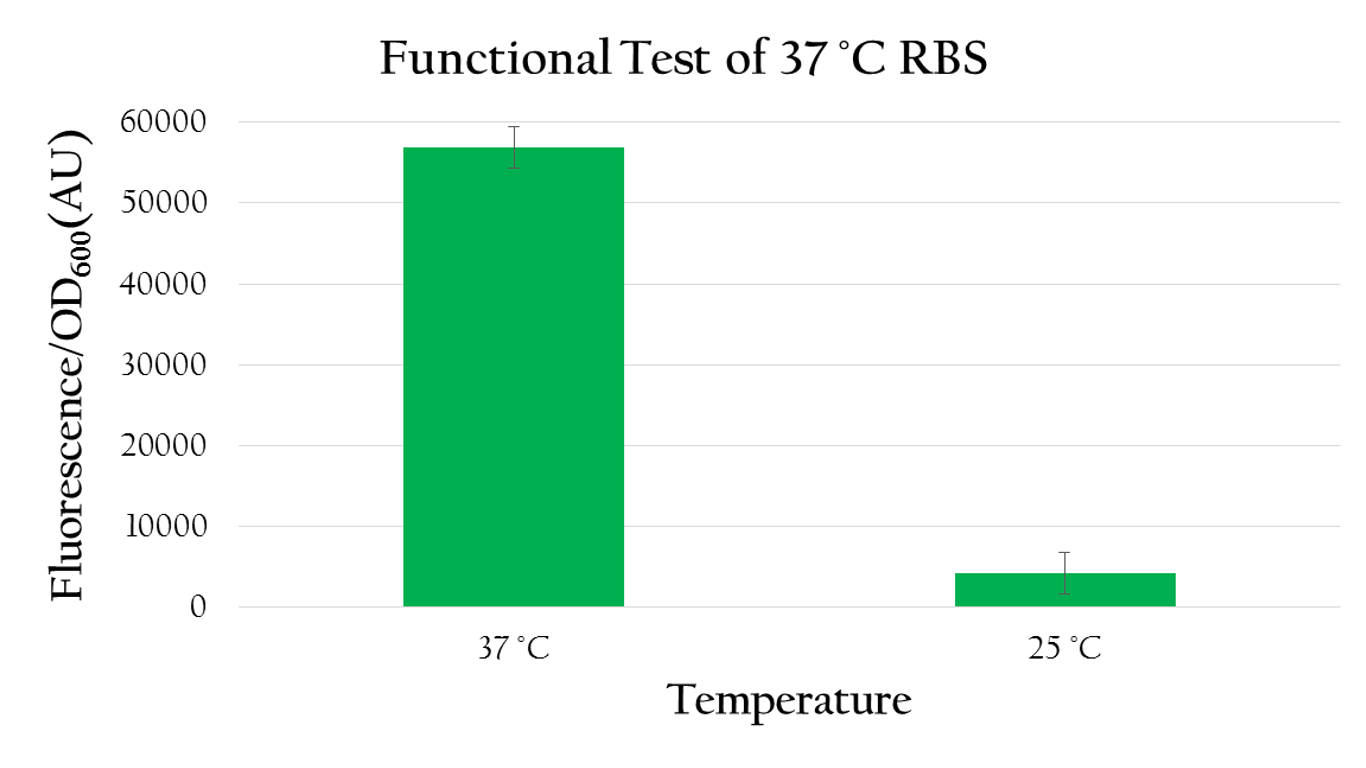
Using biobrick, Pcons+37°C RBS+mGFP+J61048, we tested the function of the 37°C RBS at room temperature (around 25°C) and at37°C.
As shown in Figure 8 the expression of GFP under 37°C is much higher than the expression under room temperature . Such result demonstrates the fact that 37°C RBS can effectively regulate gene expression by responding to temperature. The increased kinetic energy at 37°C is sufficient to cause the 37°C RBS to unfold and become available for ribosome binding. At room temperature, however, there isn't sufficient kinetic energy to unfold the hairpin structure and the structure is preserved. As a result, the translational efficiency is very low at room tempersture.
sRNA-regulated System
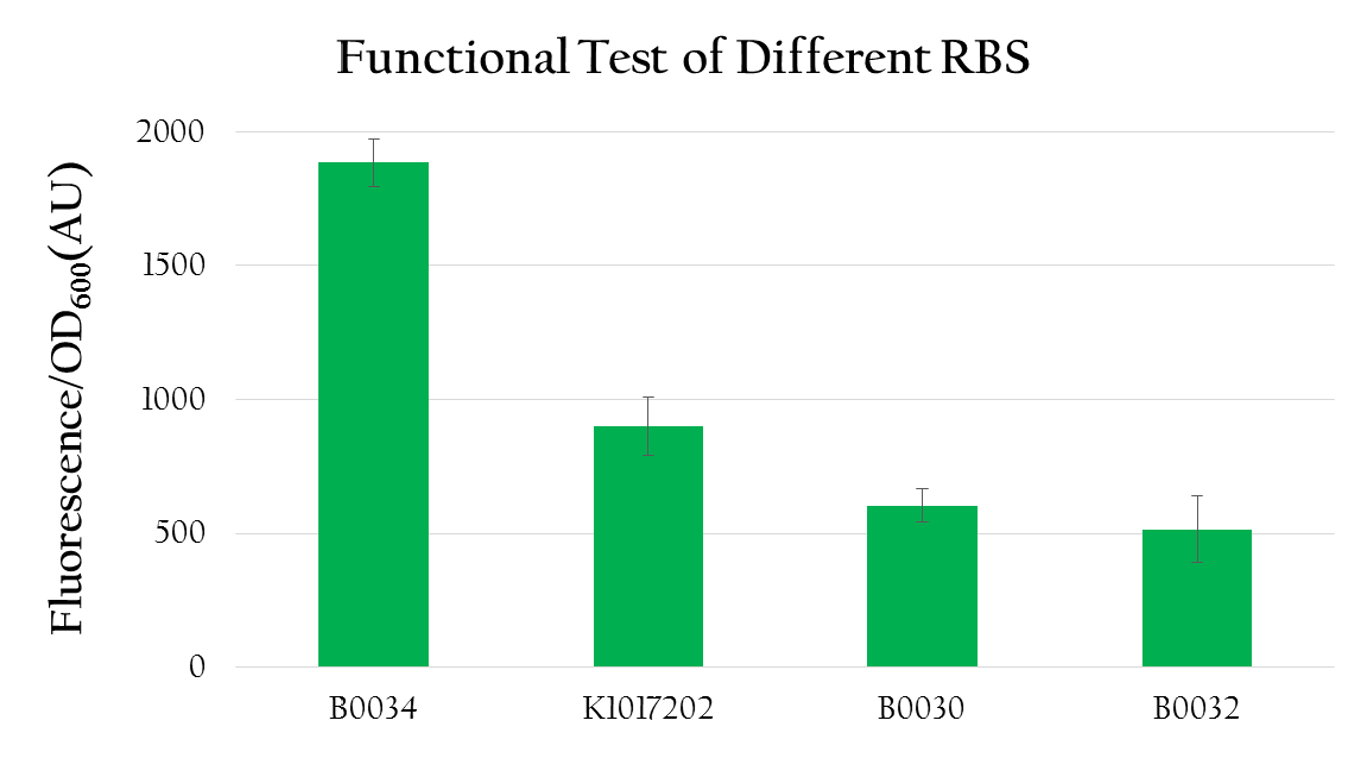
rRBS efficiency
We used the following biobrick to test the translational efficiency of K1017202 (rRBS) compared to the efficiency of other RBSs:
- Pcons+BBa_B0034+mRFP+Ter
- Pcons+[http://parts.igem.org/wiki/index.php?title=Part:BBa_K1017202 BBa_K1017202]+mRFP+Ter
- Pcons+BBa_B0030+mRFP+Ter
- Pcons+BBa_B0032+mRFP+Ter
- control: pet 30
As you can see from Figure 1, Figure 2, and Figure 3, the bacterial pellet and liquid of each biobrick shows different level of RFP expressions as the RBS of each biobrick provides a different translation efficiency. The deeper the red color is, the higher the level of expression is.
We measured the OD600 value and the florescence expression (emmision 612 nm and excitation 584 nm) for each biobrick mentioned above. The result is shown in Figure 4. We calculated the normalized expression by dividing fluorescence expression with the OD value measured, since the higher the OD value is, the larger the amount of bacteria that can express florescence would be. As shown in Figure 4, the normalized expression of the biobrick with B0034 is the highest and the one with K1017202 (rRBS) is the second highest, while the other two of B0032 and B0030 show weak expressions. This result implies that K1017202 can, in fact, serve as a functional RBS. In comparison to other RBS, K1017202 can provide moderate translational efficiency that is just lower than that of the highly efficient B0034.
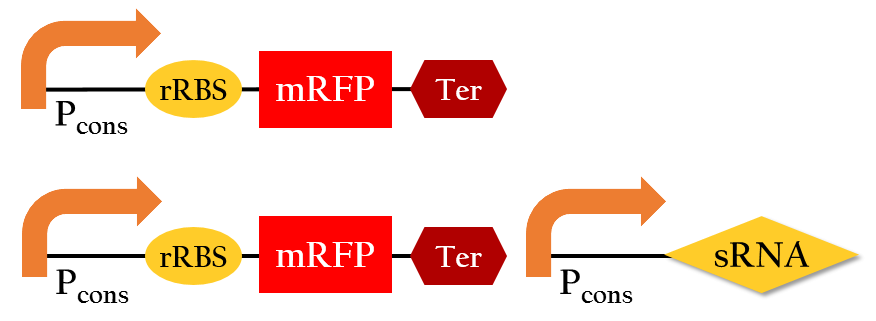
Effect on E. Coli Growth
To test whether or not our sRNA would effect the growth of E. Coli, we compared the growth of E. Coli with PSB1C3 (without RFP) and E. Coli with Pcons + sRNA. It can be observed from Figure 5 that the growth curves of the bacteria are similar, showing no signs of growth interference. This result proves that our sRNA regulated system can be integrated into bacteria.
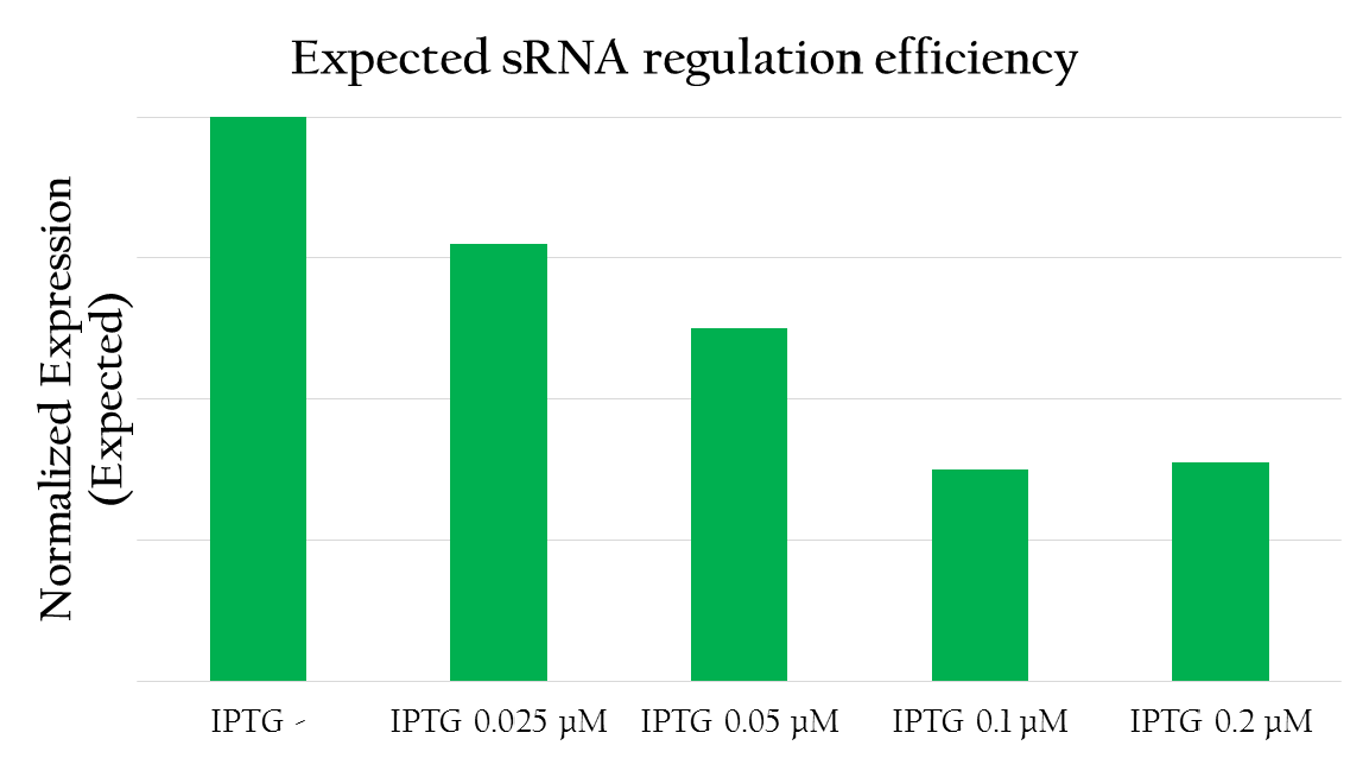
Expected sRNA regulation efficiency
We employed the following biobricks to test the regulation efficiency of the sRNA we designed: Pcons + rRBS + mGFP + J61048 and Pcons + B0030 + lacI + J61048 + Plac + sRNA.
The graph above shows the relationship between the concentration of IPTG added and the GFP expression measured. The more IPTG added, the more sRNA can be translated from Plac because IPTG serves to activate the lac promoter. In other words, the amount of sRNA and the concentration of IPTG are in a liner relationship. With more IPTG added, the amount of red fluorescent measured decreases. This implies that by increasing the amount of sRNA, we can more effectively regulate the GFP expression. sRNA, in fact, efficiently regulates the gene expression by base pairing with the rRBS.
 "
"