Team:BYU Provo/Notebook/CholeraDetection/Fallexp/Period2/Dailylog
From 2013.igem.org
| Line 102: | Line 102: | ||
[[File:9.24.13_64.jpg|250px|center|link=]] | [[File:9.24.13_64.jpg|250px|center|link=]] | ||
<br> | <br> | ||
| + | |||
| + | <font size="4"> '''9/26/13''' </font> | ||
{{TeamBYUProvoFooter}} | {{TeamBYUProvoFooter}} | ||
Revision as of 16:02, 27 September 2013
| ||
|
|
9/16/13 On Saturday we checked our bacteriophage spot test for plaques, but we saw none. Our bacteriophage lambda purification procedure failed. Dr. Grose thinks that inducing lambda to lysis by 43 degree heat shock must not have been sufficient stress. Since we know H202 does induce our phage, we're going to attempt to isolate bacteriophage lambda by doing a top agar plaque assay with a drop of hydrogen peroxide, then bathing the top agar in LB, sucking it all up and then purifying the bacteriophage by chloroform lysis procedure. Clarice has been working on submitting parts to the registry. She has cloned the Ydiv gene into the iGEM backbone to submit the part; we purified the plasmid for submission. KK, KP, CH 
9/18/13 Clarice, Kelton, Dr. Grose and I discussed our plans for the next two weeks. Clarice has been working on cloning SdiA into the iGEM backbone vector as well as GFP with a YdiV promoter. SdiA is the membrane receptor protein that recognizes cholera's AHL, and it controls the YdiV promoter. We will transform the YdiV plasmid into E.Coli and plate it next to cholera, expecting to see expression of GFP. KK,KP,CH 
9/19/13 Today we did several transformations: pIG96 into Ecoli SdiA knockout strain through electroporation, and pIG91 + SdiA ligation reaction into DH5alpha through heat shock transformation. To isolate bacteriophage lambda, we did top agar H202 plaque assays with TT9901 and TT9907 grown from frozen stock. Tomorrow we will flood the plates with LB and separate the bacteriophage. KK,KP,CH
9/20/13 The electroporation transformation of pIG96 (iGEM backbone with YdiV/GFP insert) into mutant E.Coli lacking SdiA (the membrane receptor that detects cholera's AHL) yielded many colonies, four of which we streaked onto a plate for to sequence verify the plasmid. We found the frozen strain of E.Coli K12, which we streaked today. Tomorrow we'll electroporate pIG96 into it. The Children's Book has been distributed to all team members. All team members are to find three or four families with little children to whom they can administer the pre and post test. We purified bacteriophage lambda from top agar plaque assay plates by flooding them with LB, the plates sit for 1 hour, then sucking the liquid up and using a chloroform lysis procedure to kill all remaining cells, and separation by centrifugation for 10 minutes at 8000 rpm. Spot tests will confirm tomorrow whether or not we actually isolated the bacteriophage. Clarice re-transformed pIG91+SdiA ligation into DH5alpha. Four the fourth time haha. From yesterday's transformation we saw two white colonies, which we think indicates a successful ligation of the insert into the vector, but as a safeguard, Clarice redid the transformation today. KK,KP,CH
9/23/13 Over the weekend we transformed plasmid pIG96 into E.Coli K12 via electroporation, and plated single colonies. Also, we electroporated plasmid pIG87 (with CRO insert) into TT25281. We want the latter strain to infect it with bacteriophage lambda, and then see if overexpression of CRO (which is behind the arabinose-inducible pBAD promoter) will direct bacteriophage lambda into its lytic cycle. On the subject of bacteriophage lambda, we observed that our bacteriophage purification procedure failed again. Spot tests looking for plaques from lambda did not yield any plaques. The most important observation of Saturday was E.Coli SdiA-knockout strain with transformed pIG96 plasmid, plated next to cholera, does NOT produce a green GFP response. The plasmid pIG96 contains GFP behind the YdiV promoter, which is activated by SdiA. So, we expected NOT to see a colorimetric response; thankfully, there was none. Today we started overnight cultures of TT9901 and TT9907 with lambda lysogens, to attempt once again to purify the phage. We also plated SdiA-positive E.Coli with transformed pIG96 next to cholera. We hope to see a green response tomorrow, indicating that E.Coli is detecting V.cholerae's AHL molecule through SdiA. We have divvied out assignments for our poster and presentation. We go to Canada in a week and a half! 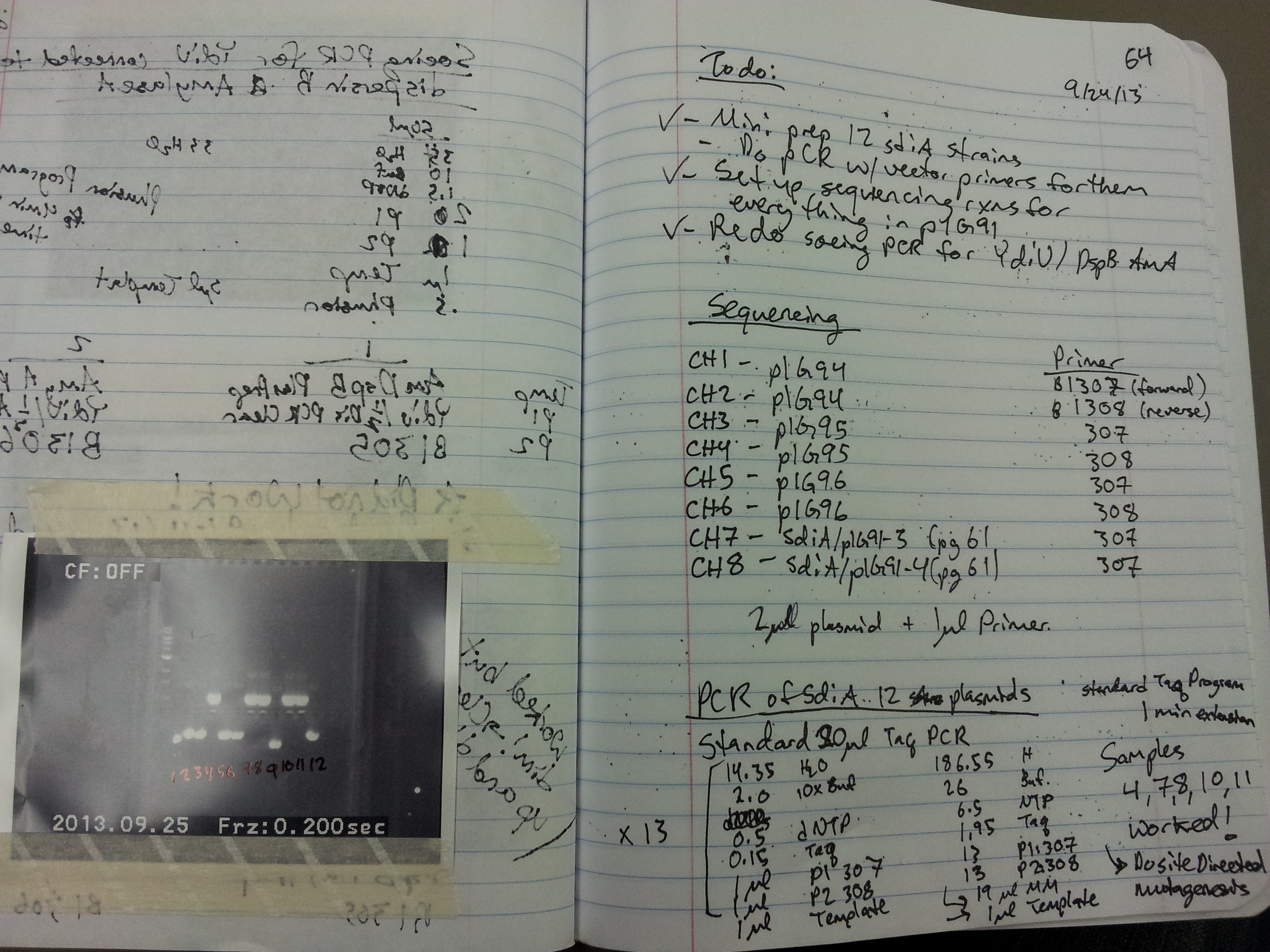
9/26/13
|
|
 "
"




