Team:BYU Provo/Results/Experimental
From 2013.igem.org
(→Designing SdiA to be Specific for Cholera) |
|||
| Line 72: | Line 72: | ||
===Designing SdiA to be Specific for Cholera=== | ===Designing SdiA to be Specific for Cholera=== | ||
| + | |||
| + | [[File:BYUK Apr10-4.JPG|250px|center|link=]] | ||
In the beginning our plan was to transform into E.coli the most essential parts of the V.cholerae quorum sensing system. This was done, but our response proteins GFP and RFP did not work as expected. So we decided to try mutating the quorum sensing system E.coli already had to be able to only sense CAI-1 which is specific to V.cholerae. | In the beginning our plan was to transform into E.coli the most essential parts of the V.cholerae quorum sensing system. This was done, but our response proteins GFP and RFP did not work as expected. So we decided to try mutating the quorum sensing system E.coli already had to be able to only sense CAI-1 which is specific to V.cholerae. | ||
Revision as of 02:16, 28 September 2013
| |
|
|
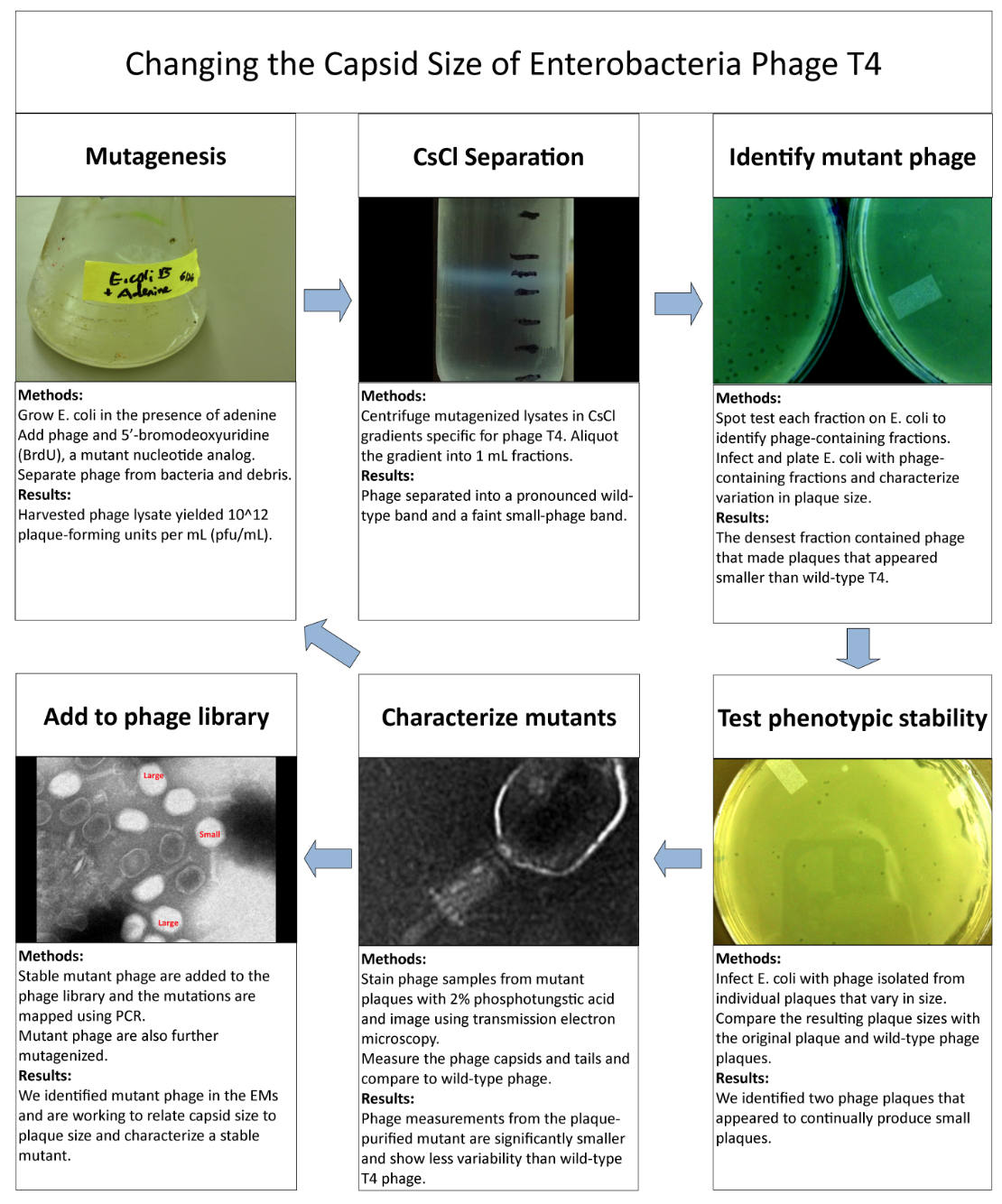
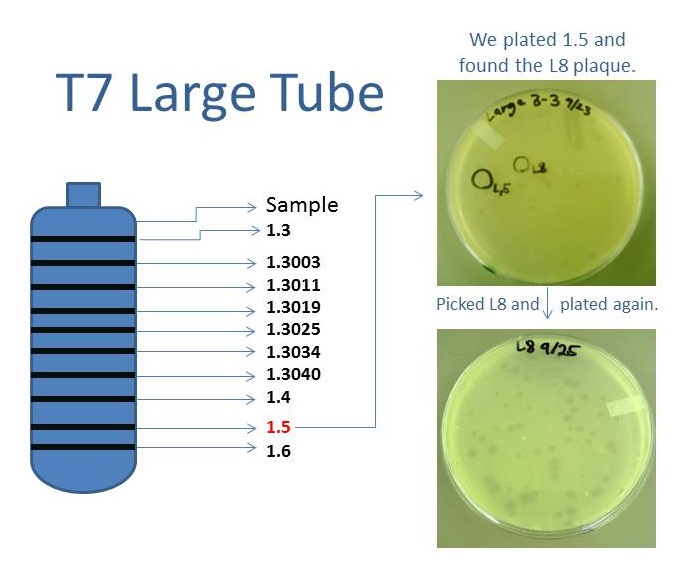
Phage TeamIsolation of Phage Library
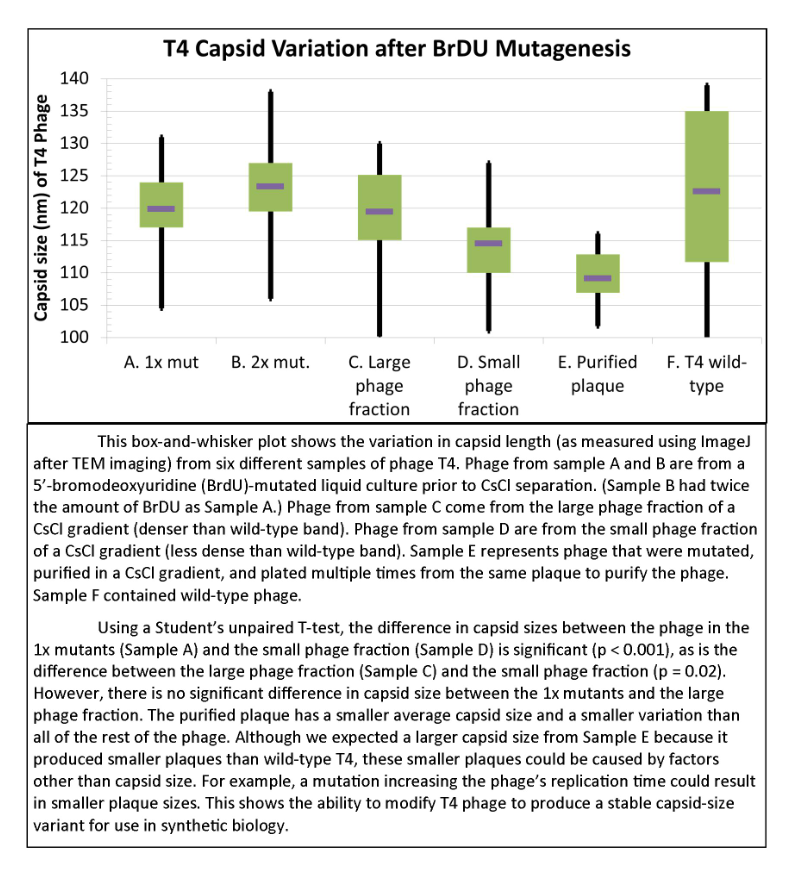
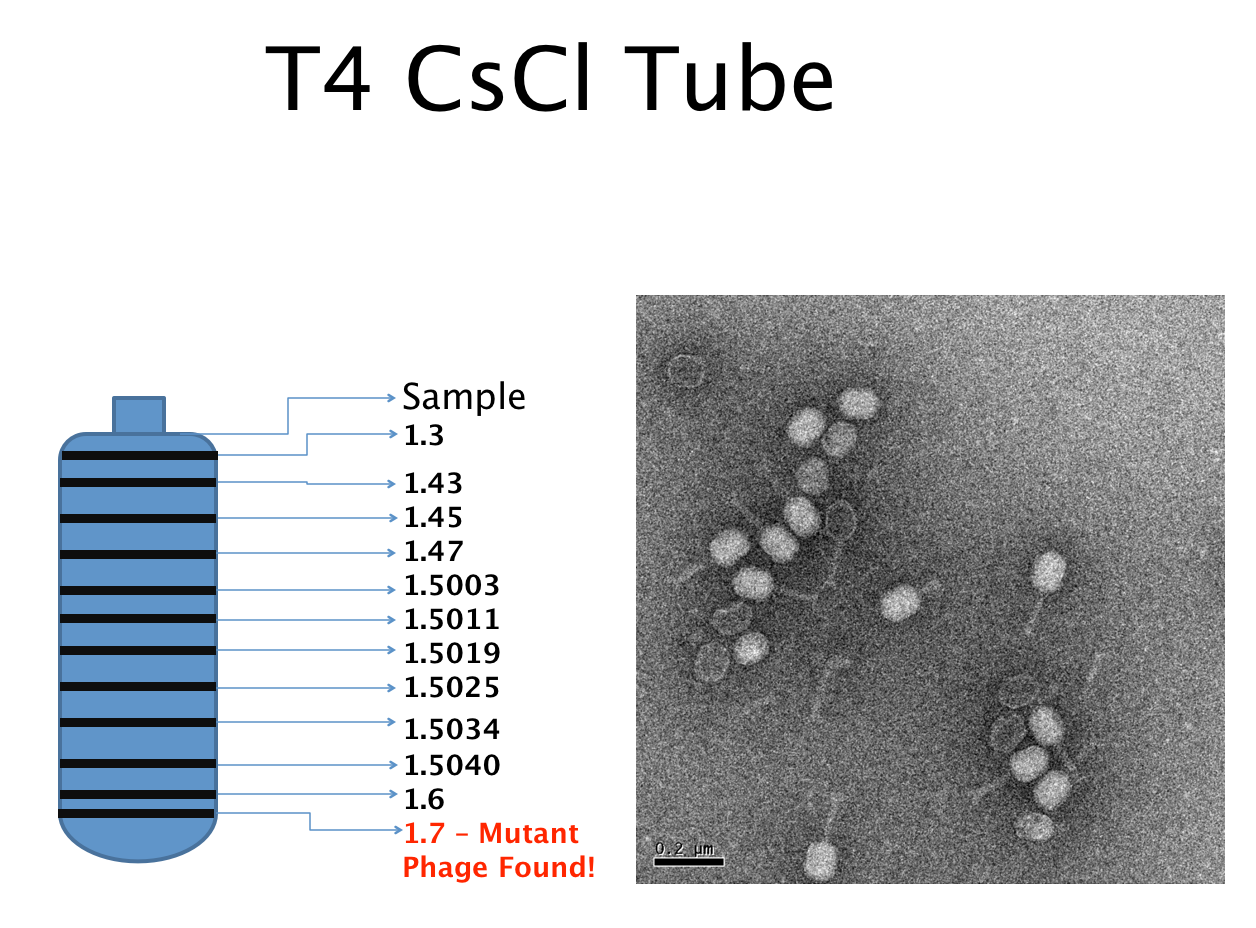
Results of Mutant T4 Phage Isolation
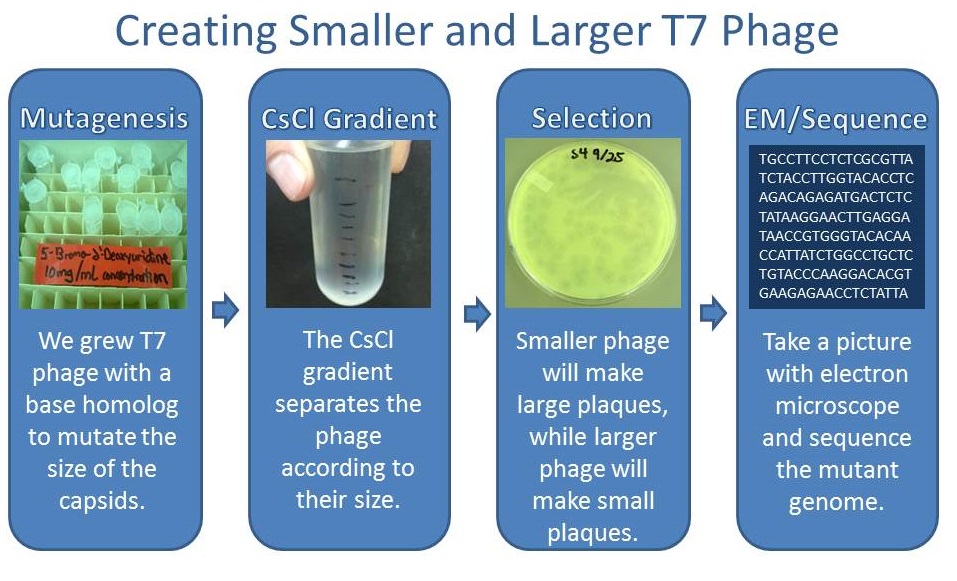
Results of Mutant T7 Phage Isolation
Cholera TeamCholera Induces Bacteriophage Lambda From Lysogeny to its Lytic Cycle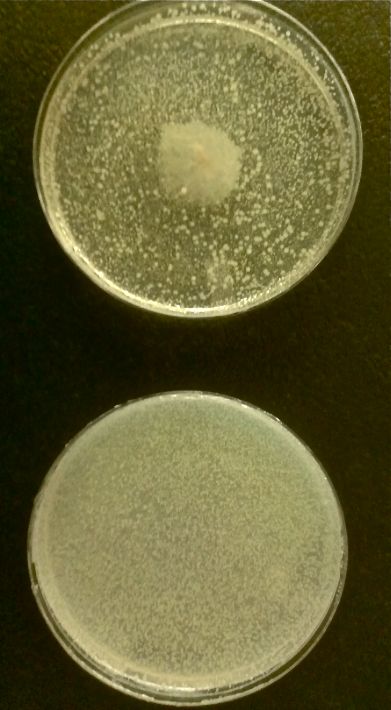
Designing SdiA to be Specific for Cholera In the beginning our plan was to transform into E.coli the most essential parts of the V.cholerae quorum sensing system. This was done, but our response proteins GFP and RFP did not work as expected. So we decided to try mutating the quorum sensing system E.coli already had to be able to only sense CAI-1 which is specific to V.cholerae. 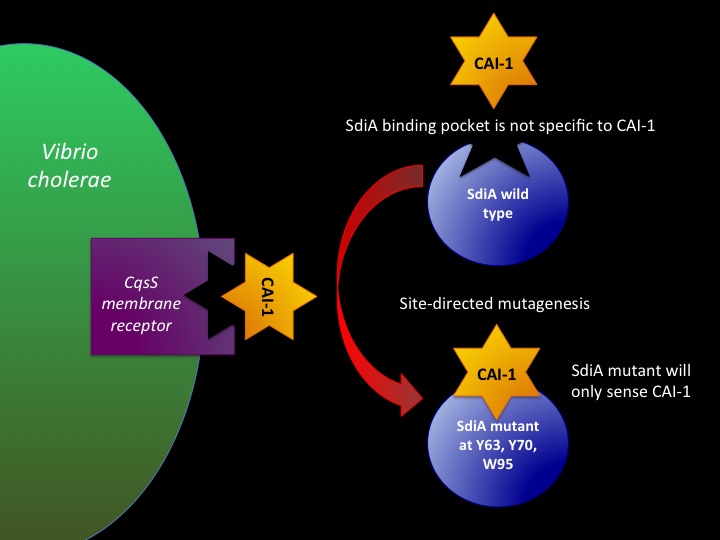
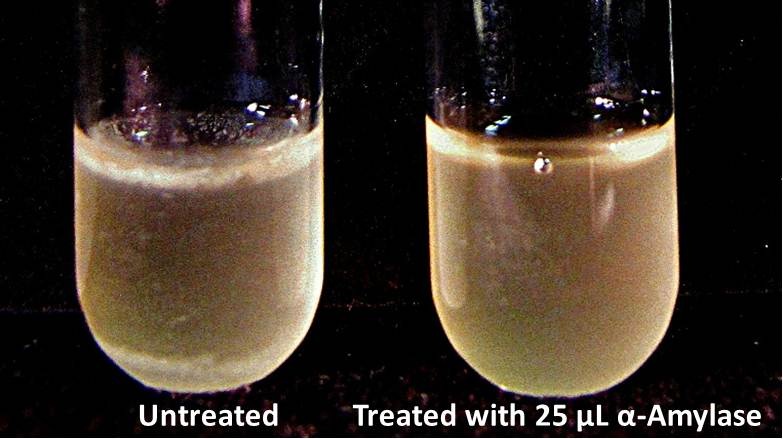
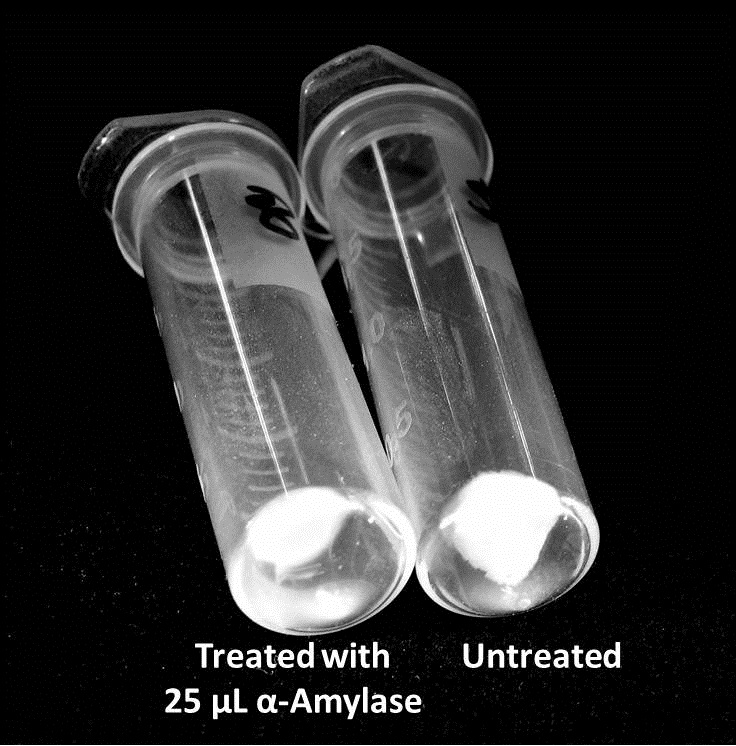
Biofilm inhibition by AmylaseThe enzymatic activity of α-Amylase was characterized to determine its capacity to inhibit biofilm formation by V. cholerae. Samples were prepared by adding 50 µL of V. cholerae culture to 1 mL of a high salt LB. The samples were then treated by the addition of purified α-Amylase in various concentrations and allowed to incubate at 30°C for 48 hours. After 48 hours the samples were examined and a distinct difference was seen in the amount of biofilm formed in the treated and untreated samples.
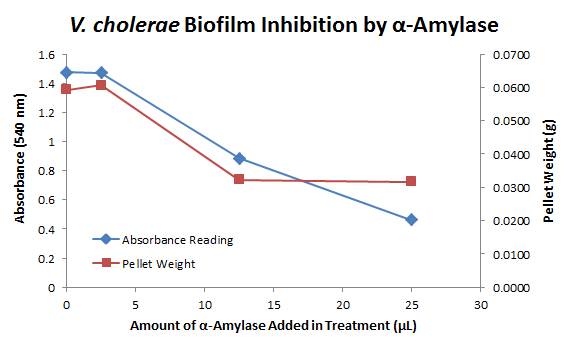
The above graph shows both the average pellet weight and the average absorbance readings for samples treated with 0, 2.5, 12.5, and 25 µL of α-Amylase. There is a distinct reduction in the amount of biofilm growth between untreated and treated samples, with samples treated with 25 µL α-Amylase showing a 65.8% decrease in biofilm formation after 48 hours. While this clearly shows the ability of α-Amylase to inhibit biofilm formation by V. cholerae, further characterization is needed to determine the capacity of α-Amylase to degrade preexisting biofilms.
|
 "
"