Team:Heidelberg/Templates/DelH week2
From 2013.igem.org
(Difference between revisions)
(→Miniprep of BioBricks) |
m |
||
| Line 1: | Line 1: | ||
| - | |||
== 06-05 - 12-05-13 == | == 06-05 - 12-05-13 == | ||
===Amplification of BioBricks=== | ===Amplification of BioBricks=== | ||
| Line 39: | Line 38: | ||
====Result==== | ====Result==== | ||
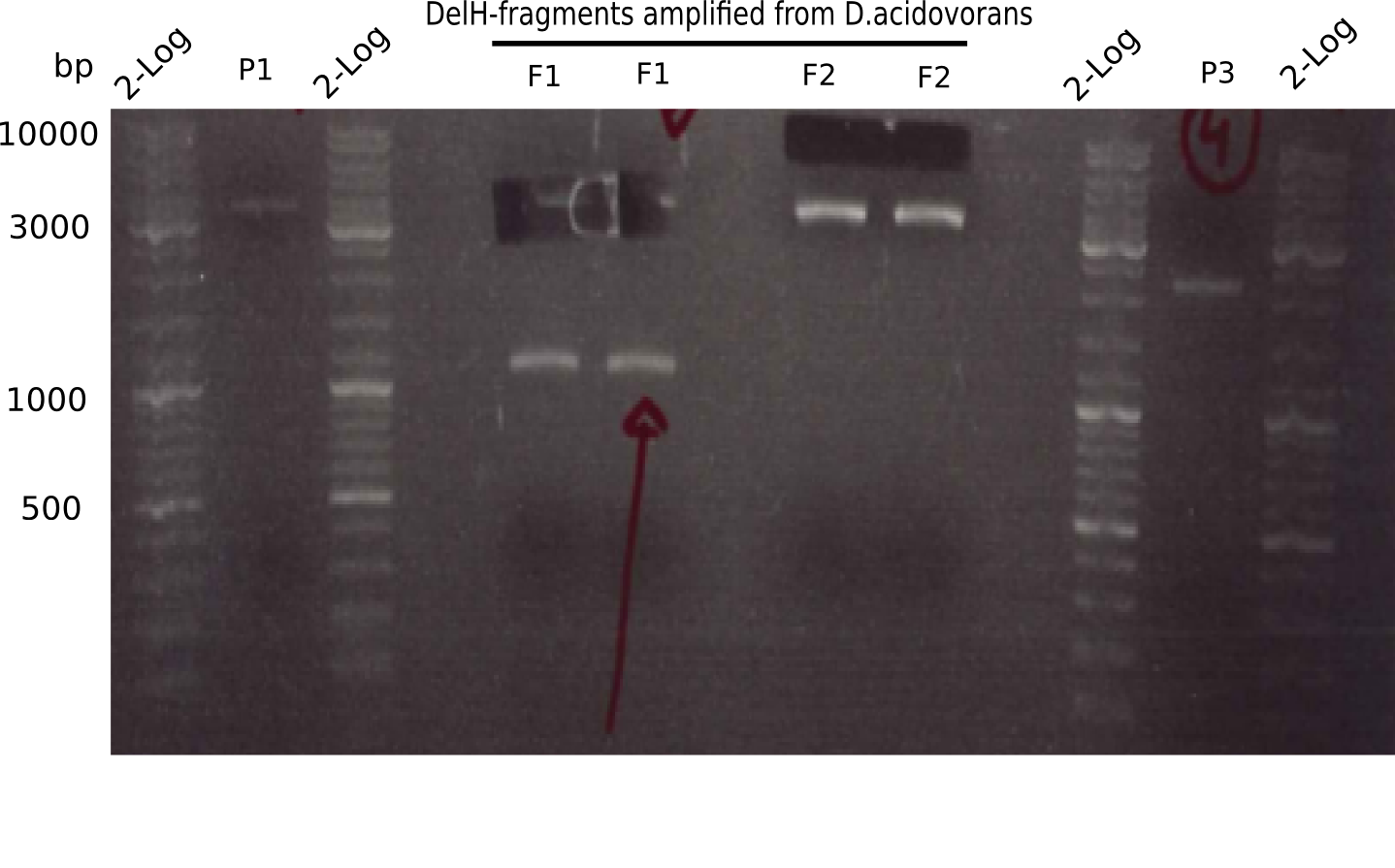
[[File:Heidelberg_20130506 2log P1 F1 F2 P3.png|200px|thumb|'''Fig. 2.1''': Restriction results of ''06-05''. ''l1:''2log, ''l2:'' P1 (pSB4K5),''l3:''2log, ''l4-5:''amplified DelH fragment 1 , ''l6-7:''amplified DelH fragment 2,''l8:''2log, ''l9:'' P3 (pSB1C3)]] | [[File:Heidelberg_20130506 2log P1 F1 F2 P3.png|200px|thumb|'''Fig. 2.1''': Restriction results of ''06-05''. ''l1:''2log, ''l2:'' P1 (pSB4K5),''l3:''2log, ''l4-5:''amplified DelH fragment 1 , ''l6-7:''amplified DelH fragment 2,''l8:''2log, ''l9:'' P3 (pSB1C3)]] | ||
| - | + | ||
<br/> | <br/> | ||
The fragments of plasmids pSB4K5 and pSB1C3 show the expected pattern. | The fragments of plasmids pSB4K5 and pSB1C3 show the expected pattern. | ||
:=> BioBrick backbone was cut and gel isolated. | :=> BioBrick backbone was cut and gel isolated. | ||
<br/> | <br/> | ||
| + | |||
| + | <div style="clear:both"></div> | ||
===Generation of LacZ Fragment=== | ===Generation of LacZ Fragment=== | ||
| Line 65: | Line 66: | ||
* The restriction mix was loaded onto a 1% agarose gel using 6 µl gene ruler marker. | * The restriction mix was loaded onto a 1% agarose gel using 6 µl gene ruler marker. | ||
[[File:Heidelberg_20130506 2log P1 F1 F2 P3.png|200px|thumb|'''Fig. 2.1''': Restriction results of ''06-05''. ''l1:''2log, ''l2:'' P1 (pSB4K5),''l3:''2log, ''l4-5:''amplified F1 for Backbone (pSB6A1), ''l6-7:''amplified fragment 2 for Backbone (lacZ),''l8:''2log, ''l9:'' P3 (pSB13)]] | [[File:Heidelberg_20130506 2log P1 F1 F2 P3.png|200px|thumb|'''Fig. 2.1''': Restriction results of ''06-05''. ''l1:''2log, ''l2:'' P1 (pSB4K5),''l3:''2log, ''l4-5:''amplified F1 for Backbone (pSB6A1), ''l6-7:''amplified fragment 2 for Backbone (lacZ),''l8:''2log, ''l9:'' P3 (pSB13)]] | ||
| - | + | ||
The fragments show the expected pattern. | The fragments show the expected pattern. | ||
:=> LacZ insert were cut and gel isolated. | :=> LacZ insert were cut and gel isolated. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
====Restriction Digest of BioBrick K173004(lacZ) Conditions B==== | ====Restriction Digest of BioBrick K173004(lacZ) Conditions B==== | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 109: | Line 110: | ||
====Result==== | ====Result==== | ||
[[File:Heidelberg_20130508 2log 2xlacZ.png|200px|thumb|right|'''Fig.2.2''' Gel of cut lacZ (loaded 20 µL) <br> , ''l1-2:'' lacz & pSB1AT,''l3:''2log ladder <br/> => lacZ was cut out]] | [[File:Heidelberg_20130508 2log 2xlacZ.png|200px|thumb|right|'''Fig.2.2''' Gel of cut lacZ (loaded 20 µL) <br> , ''l1-2:'' lacz & pSB1AT,''l3:''2log ladder <br/> => lacZ was cut out]] | ||
| - | + | ||
<br/> | <br/> | ||
:=>The band was too high on gel and discarded, because we cannot be sure if it really is lacZ or the backbone of the lacZ plasmid. | :=>The band was too high on gel and discarded, because we cannot be sure if it really is lacZ or the backbone of the lacZ plasmid. | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Generation of AraC Fragment=== | ===Generation of AraC Fragment=== | ||
====Amplification of BioBricks containing I13453 and K206000 ==== | ====Amplification of BioBricks containing I13453 and K206000 ==== | ||
| Line 164: | Line 165: | ||
<br/> | <br/> | ||
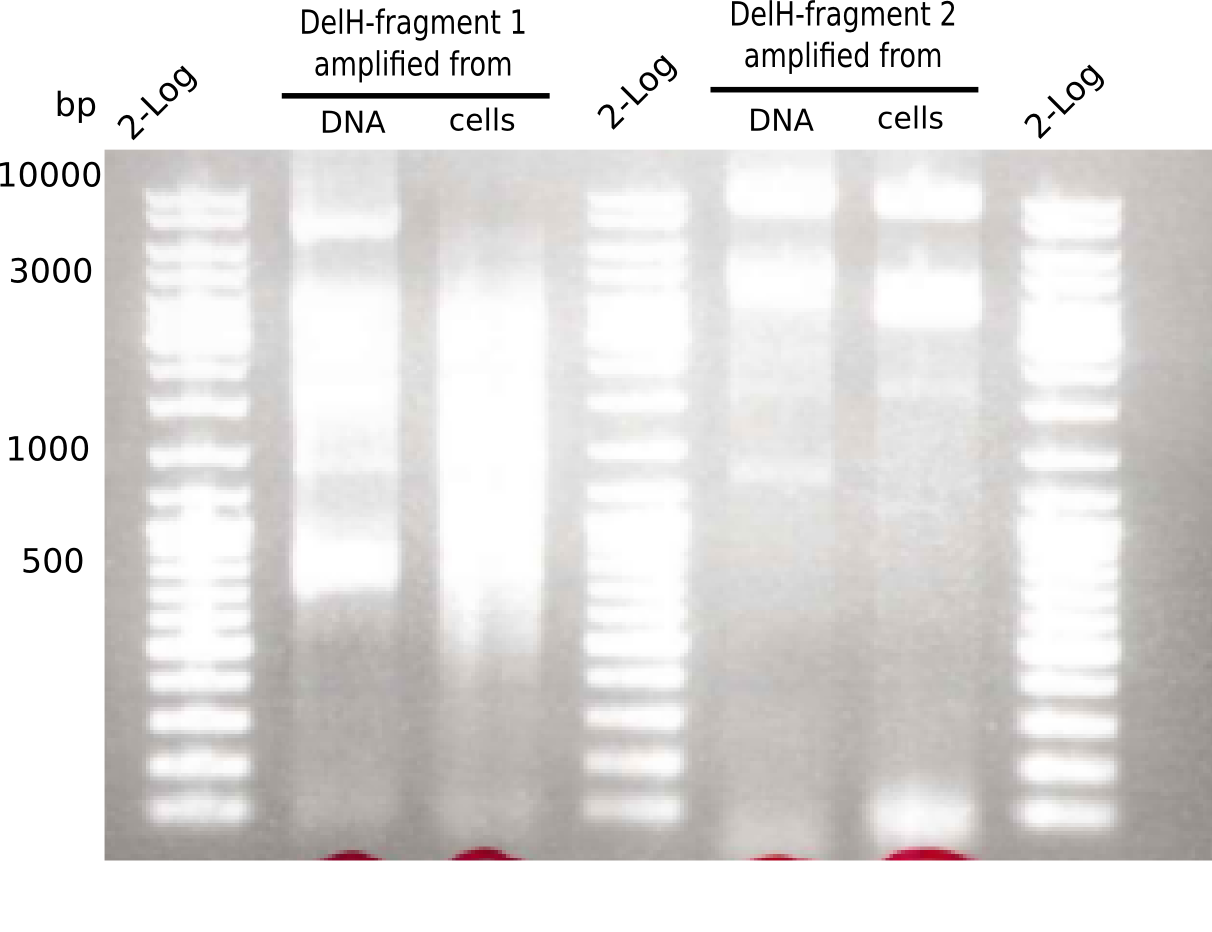
[[File:Heidelberg_20130507 2log 2xF1 2xF2.png|200px|thumb|right|'''Fig.2.3''' Gel of amplified DelH-fragment 1 & fragment 2 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2-3:''DelH-F1, ''l1:''2log ladder, ''l5-6:''DelH-F2 <br> no specific band at 10 Kb]] | [[File:Heidelberg_20130507 2log 2xF1 2xF2.png|200px|thumb|right|'''Fig.2.3''' Gel of amplified DelH-fragment 1 & fragment 2 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2-3:''DelH-F1, ''l1:''2log ladder, ''l5-6:''DelH-F2 <br> no specific band at 10 Kb]] | ||
| - | + | ||
Different unspecific bands were observed. | Different unspecific bands were observed. | ||
:=> Further optimize PCR conditions. | :=> Further optimize PCR conditions. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
====PCR Conditions F1.W2.B==== | ====PCR Conditions F1.W2.B==== | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
| Line 203: | Line 204: | ||
====Result==== | ====Result==== | ||
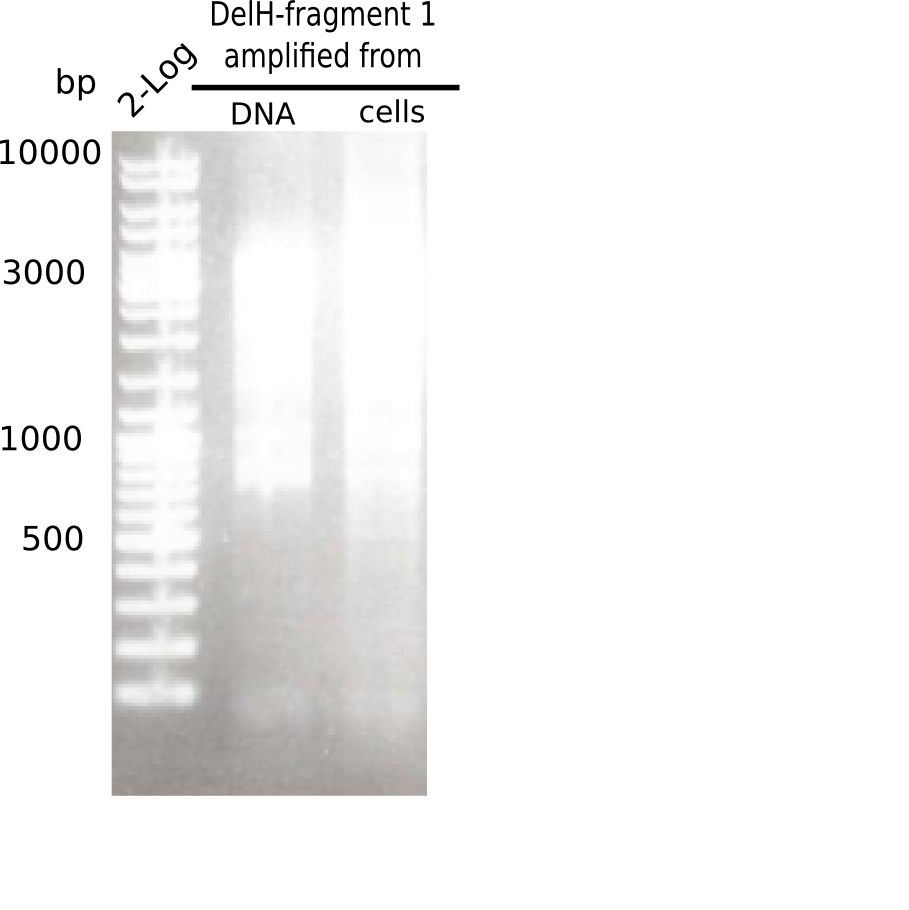
[[File:Heidelberg_20130508 2log 2xF1.png|200px|thumb|right|'''Fig.2.4''' Gel of amplified DelH-fragment 1 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2:''DelH-F1 amplified from DNA, ''l3:''DelH-F1 amplified from cells <br> no specific band at 10 Kb]] | [[File:Heidelberg_20130508 2log 2xF1.png|200px|thumb|right|'''Fig.2.4''' Gel of amplified DelH-fragment 1 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2:''DelH-F1 amplified from DNA, ''l3:''DelH-F1 amplified from cells <br> no specific band at 10 Kb]] | ||
| - | + | ||
PCRs showed only unspecific bands. | PCRs showed only unspecific bands. | ||
:=> Further optimize PCR conditions. | :=> Further optimize PCR conditions. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
====PCR Conditions F1.W2.C==== | ====PCR Conditions F1.W2.C==== | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
| Line 246: | Line 247: | ||
====Result==== | ====Result==== | ||
[[File:Heidelberg_20130510 2log 8xF1.png|200px|thumb|right|'''Fig.2.5''' Gel of DelH-fragment 1 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2-9:'' F1 with different conditions => ''l9'' shows the best match]] | [[File:Heidelberg_20130510 2log 8xF1.png|200px|thumb|right|'''Fig.2.5''' Gel of DelH-fragment 1 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2-9:'' F1 with different conditions => ''l9'' shows the best match]] | ||
| - | + | ||
PCR with DMSO and 1:10 diluted primer looked best. | PCR with DMSO and 1:10 diluted primer looked best. | ||
:=> Gel extraction performed. | :=> Gel extraction performed. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Amplification of DelH F2=== | ===Amplification of DelH F2=== | ||
====PCR Conditions F2.W2.A==== | ====PCR Conditions F2.W2.A==== | ||
| Line 296: | Line 297: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130507 2log 2xF1 2xF2.png|200px|thumb|right|'''Fig.2.3''' Gel of amplified DelH-fragment 1 & fragment 2 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2-3:''DelH-F1, ''l4:''2log ladder, ''l5-6:''DelH-F2 <br> no specific band at 8 Kb]] | [[File:Heidelberg_20130507 2log 2xF1 2xF2.png|200px|thumb|right|'''Fig.2.3''' Gel of amplified DelH-fragment 1 & fragment 2 (loaded 20 µL) <br> ''l1:''2log ladder, ''l2-3:''DelH-F1, ''l4:''2log ladder, ''l5-6:''DelH-F2 <br> no specific band at 8 Kb]] | ||
| - | + | ||
Different unspecific bands were observed. | Different unspecific bands were observed. | ||
:=> Further optimize PCR conditions. | :=> Further optimize PCR conditions. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
====PCR Conditions F2.W2.B==== | ====PCR Conditions F2.W2.B==== | ||
| Line 338: | Line 339: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130508 2log 2xF2.png|200px|thumb|right|'''Fig.2.6''' Gel of amplified DelH-fragment 1 (loaded 20 µL) <br> ''l1:''DelH-F1 amplified from DNA, ''l2:''DelH-F1 amplified from cells, ''l3:''2log ladder <br> no specific band at 8 Kb]] | [[File:Heidelberg_20130508 2log 2xF2.png|200px|thumb|right|'''Fig.2.6''' Gel of amplified DelH-fragment 1 (loaded 20 µL) <br> ''l1:''DelH-F1 amplified from DNA, ''l2:''DelH-F1 amplified from cells, ''l3:''2log ladder <br> no specific band at 8 Kb]] | ||
| - | + | ||
Gel shows the expected specific band at 8 Kb. | Gel shows the expected specific band at 8 Kb. | ||
:=> DelH F2 was thus successfully amplified from the glycerol stock. | :=> DelH F2 was thus successfully amplified from the glycerol stock. | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
Revision as of 11:27, 23 October 2013
Contents |
06-05 - 12-05-13
Amplification of BioBricks
Miniprep of BioBricks
- Miniprep was performed using Qiagen kit and eluted in 35 µl ddH2O.
- From 500 µl of liquid culture, a glycerol stock was prepared as described in Preparing glycerol stocks.
Result
- DNA concentration was determined using nanovue.
| Sample | Concentration [ng/µl] |
|---|---|
| pSB4K5 | 21 |
| pSB6A1 | 47 |
| lacZ | 45 |
| pSB1C3 | 23 |
Generation of Plasmid Backbones
Restriction Digest of BioBrick pSB6A1
| Component | Amount [µl] |
|---|---|
| PSB6A1 DNA | 32.5 |
| BSA (10x) | 5 |
| NEB2 buffer (10x) | 5 |
| Enzymes (EcoRI-HF and PstI) | 1.5 each |
| ddH2O | 4.5 |
Result
The fragments of plasmids pSB4K5 and pSB1C3 show the expected pattern.
- => BioBrick backbone was cut and gel isolated.
Generation of LacZ Fragment
Restriction Digest of BioBrick pSB1AK3 Conditions A
| Component | Amount [µl] |
|---|---|
| pSB1AK3 DNA | 32.5 |
| BSA (10x) | 5 |
| NEB3 buffer (10x) | 5 |
| Enzymes (XbaI and PstI) | 1.5 each |
| ddH2O | 4.5 |
- Incubation for 1 h at 37°C
Result
- The restriction mix was loaded onto a 1% agarose gel using 6 µl gene ruler marker.
The fragments show the expected pattern.
- => LacZ insert were cut and gel isolated.
Restriction Digest of BioBrick K173004(lacZ) Conditions B
| Component | Amount [µl] |
|---|---|
| pSB1AK3 DNA | 8.3 |
| BSA (10x) | 5 |
| NEB4 buffer (10x) | 5 |
| Enzymes (XbaI & SalI-HF) | 1.5 each |
| ddH2O | 28.7 |
- Purification was performed with Qiagen Nucleotide removal Kit and eluted in 38.5 µl ddH2O
Result
2.5 µl were measured with the Nanovue. The measurement proved the failure of miniprep.
Restriction Digest of BioBrick K173004(lacZ) Conditions C
| Component | Amount [µl] |
|---|---|
| pSB1AK3 DNA | 32.5 |
| BSA (10x) | 5 |
| NEB 3 buffer (10x) | 5 |
| Enzyme (PstI) | 1.5 |
| ddH2O | - |
Result
- =>The band was too high on gel and discarded, because we cannot be sure if it really is lacZ or the backbone of the lacZ plasmid.
Generation of AraC Fragment
Amplification of BioBricks containing I13453 and K206000
- The transformation was performed as described on 03-05 and incubated ON at 37°.
Amplification of DelH F1
PCR Conditions F1.W2.A
| Reagent | DelH F1 | DelH F1 |
|---|---|---|
| Expected length [Kb] | 10 | 10 |
| Template | 1 µl D. acidovorans glycerol stock | 1 µl D. acidovorans DNA |
| Primer 100 µM fw | 0.5 µl DelH_f1_PacI_fw | 0.5 µl DelH_f1_PacI_fw |
| Primer 100 µM rev | 0.5 µl DelH_f1_SalI_rev | 0.5 µl DelH_f1_SalI_rev |
| Phusion Master Mix (2x) | 25 µl | 25 µl |
| ddH2O | 23 µl | 23 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 16 | 98 | 1 |
| 66(↓0.5°C) | 5 | |
| 72 | 3:40 min | |
| 14 | 98 | 1 |
| 62 | 5 | |
| 72 | 3:40 min | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
Expected band: 10 Kb
Different unspecific bands were observed.
- => Further optimize PCR conditions.
PCR Conditions F1.W2.B
| Reagent | DelH-fragment1 | DelH-fragment1 |
|---|---|---|
| Expected length [Kb] | 10 | 10 |
| Template | 1 µl D. acidovorans glycerol stock | 1 µl D. acidovorans DNA |
| Primer 100 µM fw | 0.5 µl DelH_f1_PacI_fw | 0.5 µl DelH_f1_PacI_fw |
| Primer 100 µM rev | 0.5 µl DelH_f1_SalI_rev | 0.5 µl DelH_f1_SalI_rev |
| Phusion Master Mix (2x) | 25 µl | 25 µl |
| ddH2O | 23 µl | 23 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 2:45 min | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
PCRs showed only unspecific bands.
- => Further optimize PCR conditions.
PCR Conditions F1.W2.C
| Reagent | DelH F1 | DelH F1 | DelH F1 | DelH F1 |
|---|---|---|---|---|
| Expected length [Kb] | 10 | 10 | 10 | 10 |
| Template | 1 µl D. acidovorans normal | 1 µl D. acidovorans normal | 1 µl D. acidovorans 1:25 | 1 µl D. acidovorans 1:25 |
| DelH_f1_PacI_fw | 0.5 µl normal | 0.5 µl 1:10 | 0.5 µl normal | 0.5 µl 1:10 |
| DelH_f1_SalI_rev | 0.5 µl normal | 0.5 µl 1:10 | 0.5 µl normal | 0.5 µl 1:10 |
| Phusion Master Mix (2x) | 25 µl | 25 µl | 25 µl | 25 µl |
| DMSO | - | - | 2.5 µl | 2.5 µl |
| ddH2O | 23 µl | 23 µl | 20.5 µl | 20.5 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 16 | 98 | 1 |
| 62 | 5 | |
| 72 | 2:45 min | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
PCR with DMSO and 1:10 diluted primer looked best.
- => Gel extraction performed.
Amplification of DelH F2
PCR Conditions F2.W2.A
| Reagent | DelH F2 | DelH F2 |
|---|---|---|
| Expected length [Kb] | 8 | 8 |
| Template | 1 µl D. acidovorans glycerol stock | 1 µl D. acidovorans DNA |
| Primer 100 µM fw | 0.5 µl DelH_f2_SalI_fw | 0.5 µl DelH_f2_SalI_fw |
| Primer 100 µM rev | 0.5 µl DelH_f2_KpnI_rev | 0.5 µl DelH_f2_KpnI_rev |
| Phusion Master Mix (2x) | 25 µl | 25 µl |
| ddH2O | 23 µl | 23 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 16 | 98 | 1 |
| 66(↓0.5°C) | 5 | |
| 72 | 3:40 min | |
| 14 | 98 | 1 s |
| 62 | 5 s | |
| 72 | 3:40 min | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
Expected band: 8 Kb
Different unspecific bands were observed.
- => Further optimize PCR conditions.
PCR Conditions F2.W2.B
| Reagent | DelH F2 | DelH F2 |
|---|---|---|
| Expected length [Kb] | 8 | 8 |
| Template | 1 µl D. acidovorans glycerol stock | 1 µl D. acidovorans DNA |
| Primer 100 µM fw | 0.5 µl DelH_f2_SalI_fw | 0.5 µl DelH_f2_SalI_fw |
| Primer 100 µM rev | 0.5 µl DelH_f2_KpnI_rev | 0.5 µl DelH_f2_KpnI_rev |
| Phusion Master Mix (2x) | 25 µl | 25 µl |
| ddH2O | 23 µl | 23 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 s |
| 72 | 2:45 min | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Results
Expected band: 8 Kb
Gel shows the expected specific band at 8 Kb.
- => DelH F2 was thus successfully amplified from the glycerol stock.
 "
"