Team:Tianjin/Project/Design&Construction
From 2013.igem.org
Desmondlee (Talk | contribs) (→a. what's the influence of the high leakage) |
Desmondlee (Talk | contribs) (→Reconstruction) |
||
| Line 359: | Line 359: | ||
We use a weaker promoter to control the expression of AlkR. And then, to reduce the interference of two transcription unit, we use a stronger terminator to the downstream of two transcription unit. | We use a weaker promoter to control the expression of AlkR. And then, to reduce the interference of two transcription unit, we use a stronger terminator to the downstream of two transcription unit. | ||
| - | At last, we switch the position of the two transcription unit, to guarantee that PalkM-RFP isn’t interferenced by constitutive promoter. The new reconstructed Alk-Sensor shows in Figure 5 | + | At last, we switch the position of the two transcription unit, to guarantee that PalkM-RFP isn’t interferenced by constitutive promoter. The new reconstructed Alk-Sensor shows in Figure 5. |
| - | At last, we use full dose octane to induce the two sensors. After a certain period of time of cultivation, the relative fluorescent intensity was measured. The results show that after reconstruction the leakage decreases by 50%, the dynamic range increases by 5 folds, which means that the optimized AlkSensor has stronger ability to distinguish different inputs. ( showed in Figure 6) | + | [[Image:TJU-PROJECT-D5.jpg.png|thumb|500px|center|<b>Figure5-1.</b> AlkSensor after reconstruction(Changed the promoter of ALKR to a weaker promoter.Switch the position of gene pieces ALKR and PalkM-RFP. Replace terminator B1006 with a stronger terminator, B0015)]] |
| + | |||
| + | [[Image:TJU-PROJECT-D5-2.png|thumb|600px|center|<b>Figure5-2.</b> Comparison of two versions of AlkSensor ,Visible result of red colony decreased on LB solid plate(up),the Statistical results of red colony and white colony.]] | ||
| + | |||
| + | Through another blank test we found that the reconstruction could decrease AlkSensor’s leakage significantly. After optimization, the percent of bacterial colonies are red enough to be distinguished by naked eyes decrease from 95% to 15%. | ||
| + | |||
| + | At last, we use full dose octane to induce the two sensors. After a certain period of time of cultivation, the relative fluorescent intensity was measured. The results show that after reconstruction the leakage decreases by 50%, the dynamic range increases by 5 folds, which means that the optimized AlkSensor has stronger ability to distinguish different inputs. ( showed in Figure 6) | ||
| + | |||
| + | [[Image:TJU-PROJECT-D6.jpg|thumb|500px|center|<b>Figure6.</b> Octane induce test of two versions of AlkSensor Original Design without octane (left 1) Original Design with octane (left 2) Reformed Design without octane (left 3) Reformed Design without octane (left 4).]] | ||
So we choose this construction as our final design for Alk-Sensor and one of our favorite biobrick. | So we choose this construction as our final design for Alk-Sensor and one of our favorite biobrick. | ||
| Line 372: | Line 380: | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| - | <a href="https://2013.igem.org/Team:Tianjin/Project/Introduction"><img src="https://static.igem.org/mediawiki/2013/d/d3/TJU-project-banner01.jpg" width=" | + | <a href="https://2013.igem.org/Team:Tianjin/Project/Introduction"><img src="https://static.igem.org/mediawiki/2013/d/d3/TJU-project-banner01.jpg" width=" |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 21:33, 28 October 2013
Contents |
First Trail
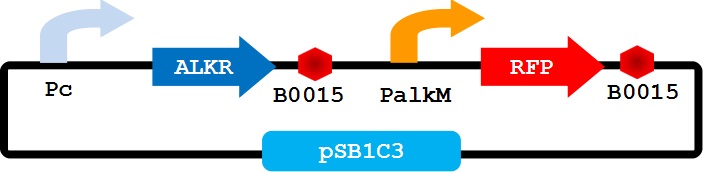
To utilize the special alkane sensing system AlkR-PalkM of Acinetobacter baylyi ADP1 in E. coli, we built a gene circus as Figure 1 showed. We use a constitutive promoter to control the expression of regulator AlkR, and RFP is controlled by PalkM. Without alkane, the PalkM will not work, and RFP will not be expressed. When alkanes exist the AlkR dimmer will bind the alkanes molecular to form a complex, the complex can turn on the expression of RFP which is controlled by PalkM. The RFP can be detected and measured by naked eyes or fluorescence spectrophotometer easily. Considering another modular may be used for E. coli to produce alkane, we put Pc-AlkR-tt and PalkM-RFP-tt into one plasmid to form our Alk-Sensor.
[[Image:#.png|thumb|400px|center|Figure1.]]
Unfortunately, our first trial has a big problem: the E. coli with our Alk-Sensor is red even in the situation without alkane, as Figure 2 shows. The leakage of the expression of our Alk-Sensor is very high. This will influence alkane detected by naked eyes, And the Alk-Sensor need to be improved.
Analysis
a. what's the influence of the high leakage
To find the source of the problem, we built a model for the process of alkane sensing of AlkR-PalkM system. (see more details for the process of modeling) The modeling result which reflects the relationship between input (alkane concentration) and output (RFP concentration) has been showed in Figure 3. From the Figure, we find that, when leakage of the AlkR-PalkM system is very high, the dynamicrange will be small. This will make it hard for us to distinguish the change of the alkane concentration, so it is important for improving the function of our Alk-Sensor to reduce the leakage.
b. what cause the leakage (from modeling information)
[[Image:#.png|thumb|400px|center|Figure3.]]
From the equation 1, which is gotten from mathematical analyse, we can find the source of leakage. In the equation, the [Pmt] and [AlkR] can be easily controlled through changing the copy number of the plasmid or strength of promoter, so we choose these two factors as our target to improve our Alk-Sensor.
To evaluate the influence of two factors, we test the parts PalkM-RFP in a high copy number plasmid pSB1C3 without the expression of AlkR. The result shows in Figure 4, we can see that the strain just containing PalkM-RFP isn’t red, and the strain with both PalkM-RFP and Pc-AlkR turns red. So the leakage is mainly caused by the high expression of AlkR and PalkM is a very weak promoter without the induction of AlkR.
[[Image:#.png|thumb|400px|center|Figure4.]]
Besides the high expression of AlkR, the low activity of the terminator downstream the AlkR also the source of leakage. In this condition, the transcription of constitutive promoter Pc may turn on the expression of RFP.
Summary of the leakage of RFP:
a)Promoter alkM has some leakage, to be more specific, it binds to RNA polymerase without undergoing a conformation change and activates the transcription. b)Protein ALKR or dimerized ALKR could induce promoter alkM without alkane molecules. c)The terminator downstream AlkR is weak the constitutive promoter may influence the expression of RFP.
Reconstruction
To improve the function of the Alk-Sensor and decrease the leakage, we reconstructed our device.
We use a weaker promoter to control the expression of AlkR. And then, to reduce the interference of two transcription unit, we use a stronger terminator to the downstream of two transcription unit.
At last, we switch the position of the two transcription unit, to guarantee that PalkM-RFP isn’t interferenced by constitutive promoter. The new reconstructed Alk-Sensor shows in Figure 5.
Through another blank test we found that the reconstruction could decrease AlkSensor’s leakage significantly. After optimization, the percent of bacterial colonies are red enough to be distinguished by naked eyes decrease from 95% to 15%.
At last, we use full dose octane to induce the two sensors. After a certain period of time of cultivation, the relative fluorescent intensity was measured. The results show that after reconstruction the leakage decreases by 50%, the dynamic range increases by 5 folds, which means that the optimized AlkSensor has stronger ability to distinguish different inputs. ( showed in Figure 6)
So we choose this construction as our final design for Alk-Sensor and one of our favorite biobrick.
 "
"




 Retrieved from "
Retrieved from "