Team:BYU Provo/Notebook/SmallPhage/Springexp/Period1/PR
From 2013.igem.org
(Difference between revisions)
(Created page with "{{TeamBYUProvo}} <br> {| width="100%" | colspan="3" | <font color="#333399" size="5" font face="Calibri"> '''Small Phage Spring Notebook: April 29 - May 12 Progress Report'''</...") |
|||
| Line 41: | Line 41: | ||
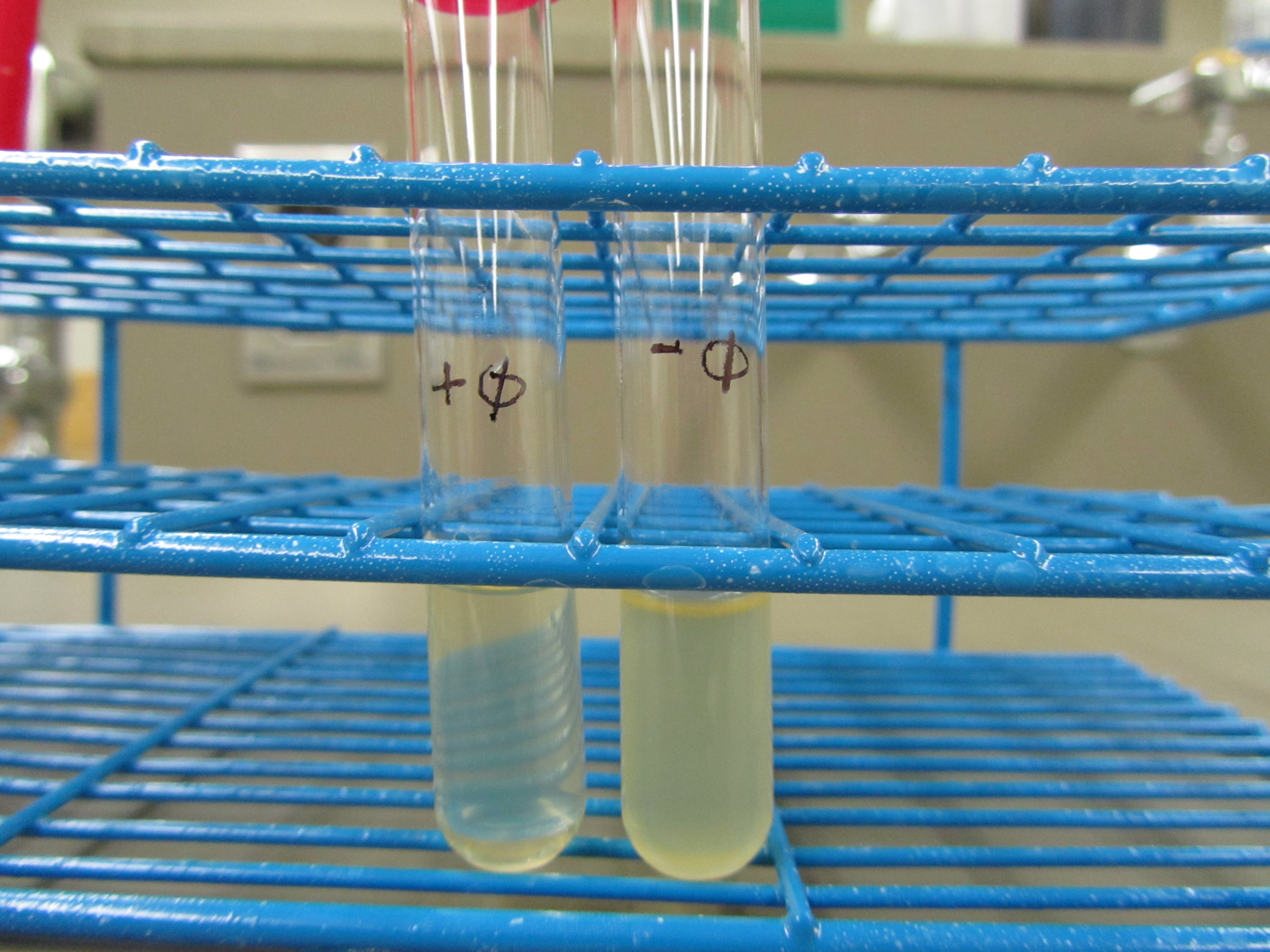
:: - phage: 4mL LB + 1mL E. coli overnight | :: - phage: 4mL LB + 1mL E. coli overnight | ||
| - | : [[File:SpringPR1P1 | + | : [[File:SpringPR1P1.JPG|300px|left]] |
:: Obvious clearage was seen for the + phage test tube | :: Obvious clearage was seen for the + phage test tube | ||
: Spot test result | : Spot test result | ||
:: We purified the phage via centrifugation and adding chloroform. This batch of phage is stored as stock 5.3 in the fridge. | :: We purified the phage via centrifugation and adding chloroform. This batch of phage is stored as stock 5.3 in the fridge. | ||
| - | : [[File:SpringPR1P2 | + | : [[File:SpringPR1P2.JPG|300px|left]] |
:: This confirmed our conclusion that we are able to grow T7 in liquid culture with E. coli BL21. | :: This confirmed our conclusion that we are able to grow T7 in liquid culture with E. coli BL21. | ||
<br> | <br> | ||
| Line 67: | Line 67: | ||
: [[File:SpringPR1P5,JPG|300px|left]] | : [[File:SpringPR1P5,JPG|300px|left]] | ||
:: However, concentrated top agar solidify so fast that we weren’t able to get it even spread on the plate. We decided to try warming up the plates in 37°C incubator for 3 hours before use (5.9 T7 Phage Selection Method Test #2). Our plates are much prettier this time. | :: However, concentrated top agar solidify so fast that we weren’t able to get it even spread on the plate. We decided to try warming up the plates in 37°C incubator for 3 hours before use (5.9 T7 Phage Selection Method Test #2). Our plates are much prettier this time. | ||
| - | : [[File:SpringPR1P6 | + | : [[File:SpringPR1P6.JPG|300px|left]] |
<br> | <br> | ||
| Line 73: | Line 73: | ||
: We wanted to see whether or not we can grow liquid culture from a single plaque. | : We wanted to see whether or not we can grow liquid culture from a single plaque. | ||
:: 4mL LB + 1mL E. coli overnight + 1 stab at a plaque from a ×6 plate in the 5.3 T7 Phage Selection Method Test. The plaque in question is circled in the photo above. After incubation for approximately 24 hours, phage solution was purified and spot test was performed. The remaining phage solution is labeled stock 5.4 in the fridge. | :: 4mL LB + 1mL E. coli overnight + 1 stab at a plaque from a ×6 plate in the 5.3 T7 Phage Selection Method Test. The plaque in question is circled in the photo above. After incubation for approximately 24 hours, phage solution was purified and spot test was performed. The remaining phage solution is labeled stock 5.4 in the fridge. | ||
| - | : [[File:SpringPR1P7 | + | : [[File:SpringPR1P7.JPG|300px|left]] |
<br> | <br> | ||
Revision as of 16:54, 27 May 2013
| Small Phage Spring Notebook: April 29 - May 12 Progress Report
| ||
|
Overview
|
1. Goals for the week
2.Experiments and results Phage growth in liquid culture (5.3 Phage Amplification/Purification)
Amplifying phage (5.6 T7+ Liquid Culture Phage Concentration Test)
Selection test
Stabbing test (5.5 Amplification from a plaque test)
Others
3. Next Steps
| |
 "
"