Team:BYU Provo/Notebook/LargePhage/Springexp/Period3/PR
From 2013.igem.org
PROGRESS REPORT
Contents |
Goals
Run a dilution series to test the titer of our mutated phage stock. Start selecting for small plaques and learning how to pick them and titer them out. Run multiple UV tests and compare mutated phage stock with the normal stock.
Accomplishments
Progress report stuff: Summary of what we did:
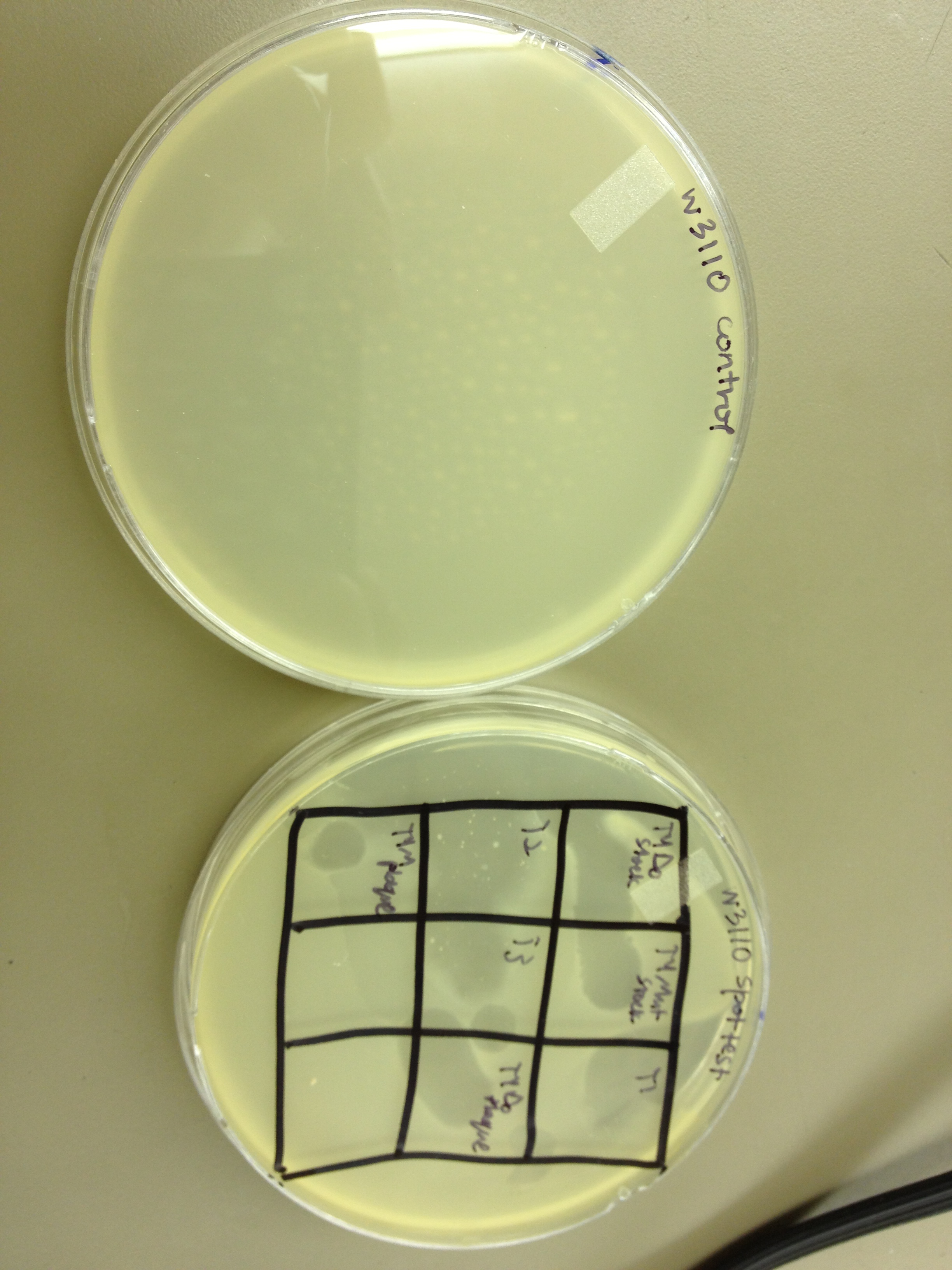
During this period we began with T4Do stock and grew it in liquid culture with 5-bromodeoxyuridine to mutagenize it. Then, this mutagenized stock as well as the standard T4Do stock were subjected to UV light in a BSL-2 hood in Dr. Breakwell’s lab. We found that it takes about 9 minutes to destroy all or almost all of the phage in a 20 uL drop on parafilm in the center of the hood. We tried to then select small plaques and culture them to see if they would remain small, but we saw no such trends. We ran a titer on the mutagenized phage stock but saw no indications that the phage actually infected the bacteria and propagated; instead, it appears that the stock may have just been diluted. This would explain why both seemed to behave similarly after being irradiated with UV light. After we received E. coli B, we tested whether all of our phage stocks will still infect this slightly different host. All of our phage showed a positive result on the spot tests, but for some reason the titer for T1 did not work. T4Do produced plaques on E. coli B just as it did on W3110. We also did some EM work with the smallest plaque found on the T4Do stock after 7.5 min of UV exposure and the smallest plaque found on the T4 mutagenized stock after 7.5 min of UV exposure. Due to the preparation of the grids or problems with the samples, the initial results did not show full phage. Both showed a lot of capsids only. We will wait until we receive the scanned images from the film and will analyze our findings then.
Began Mutagenesis with UV light
UV test-1
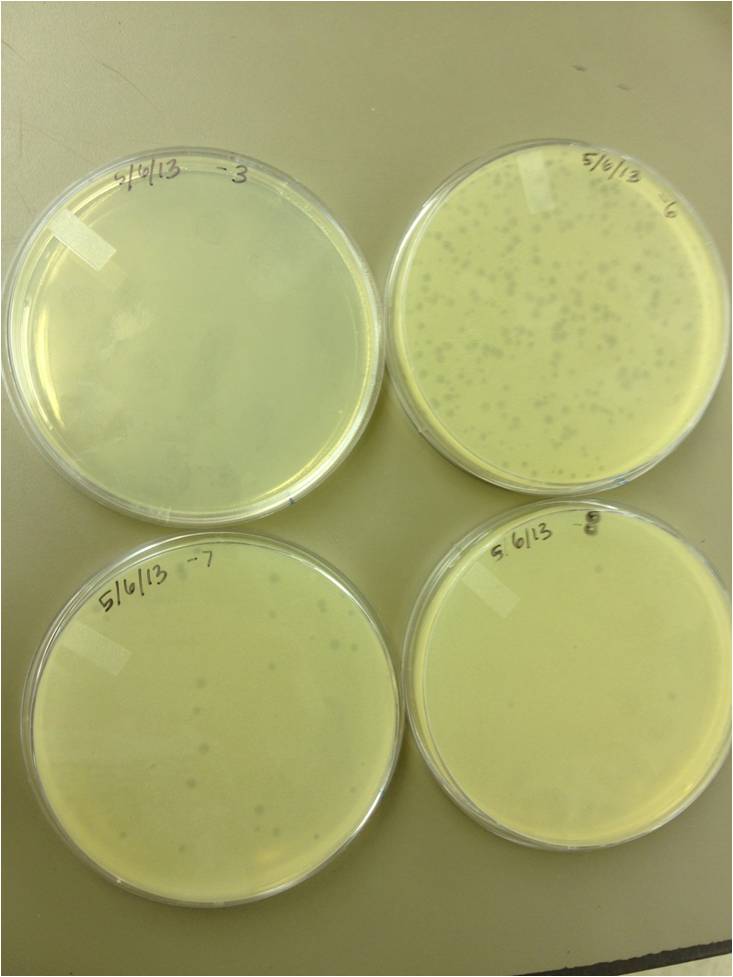
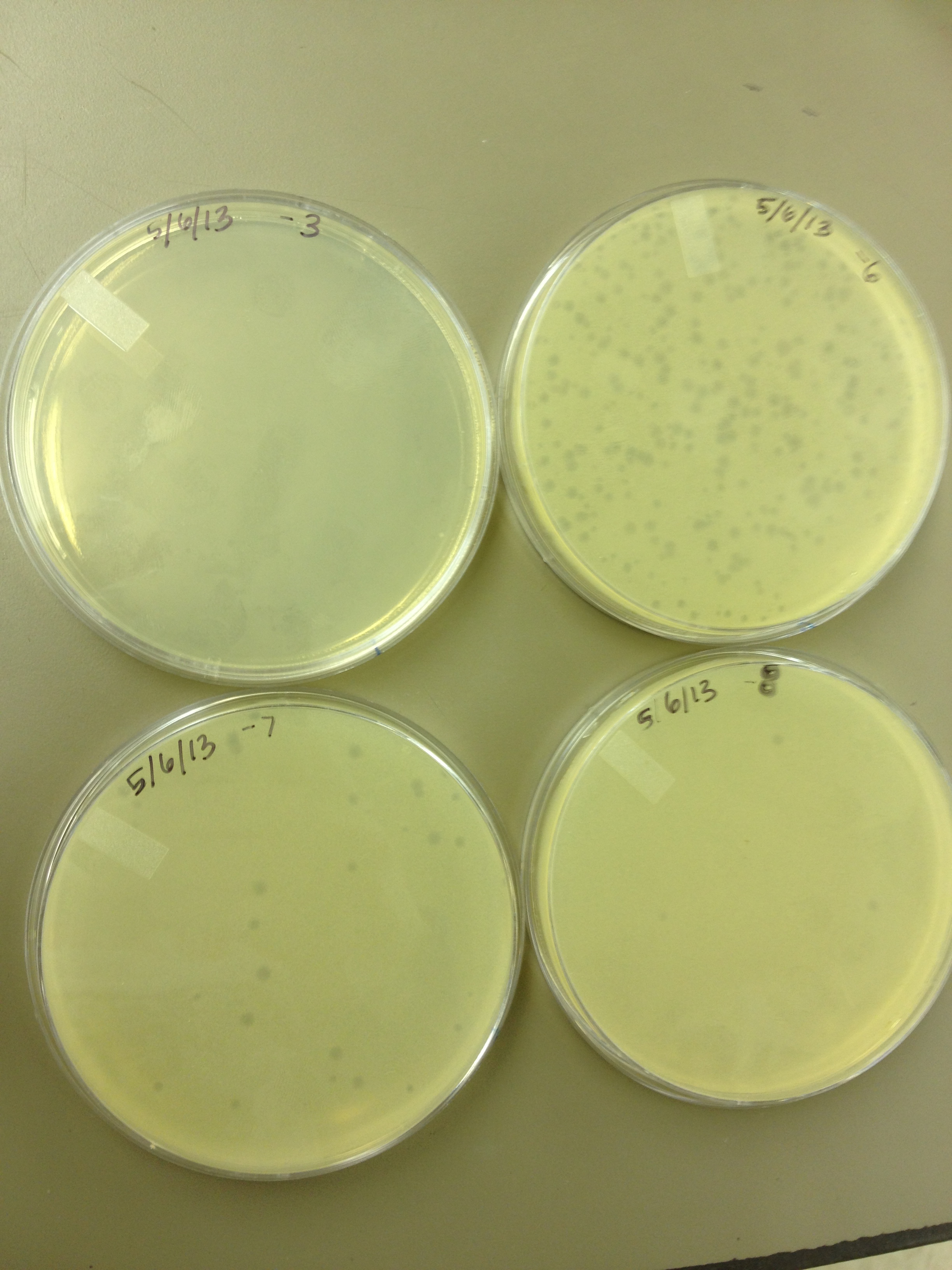
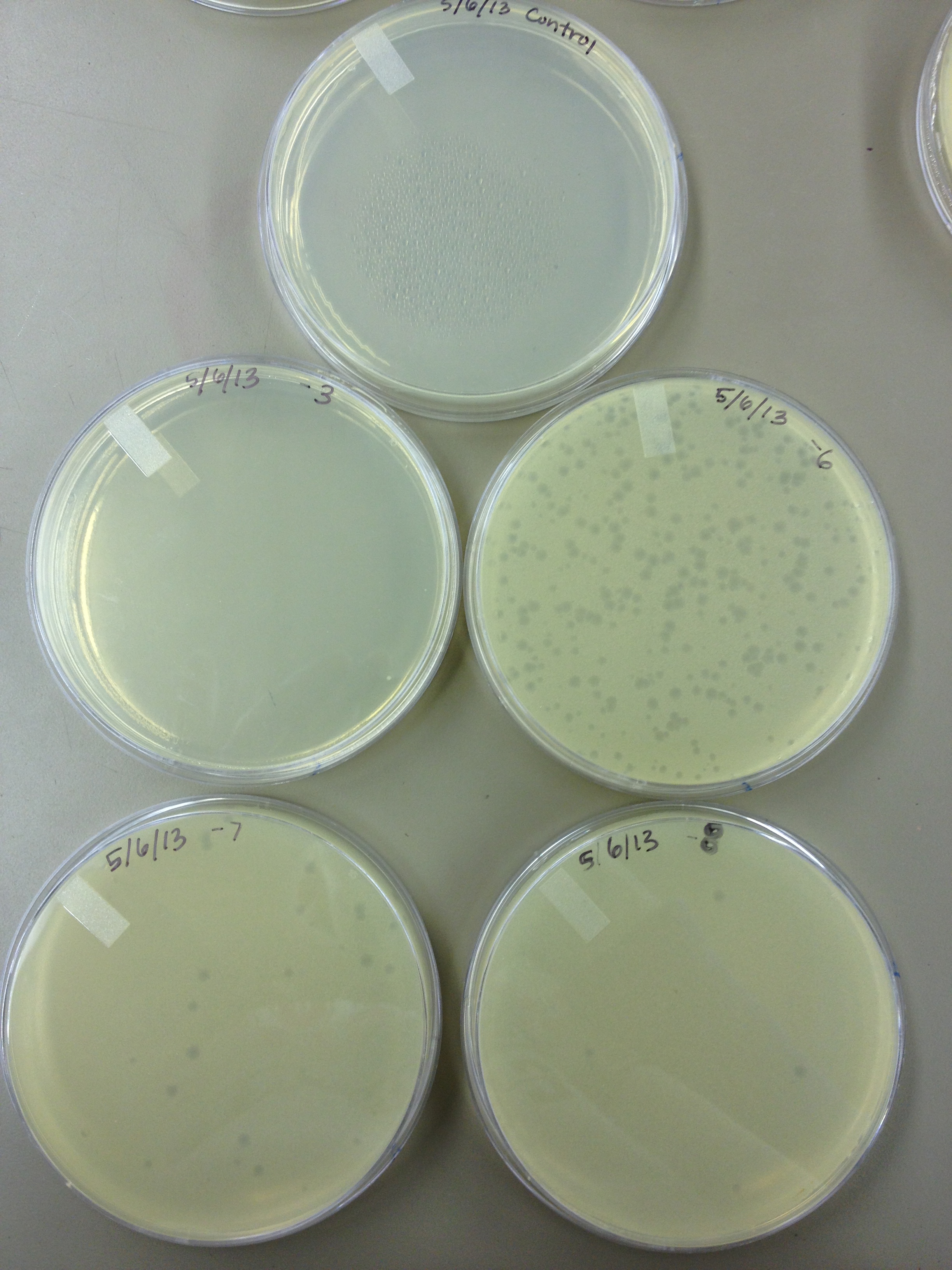
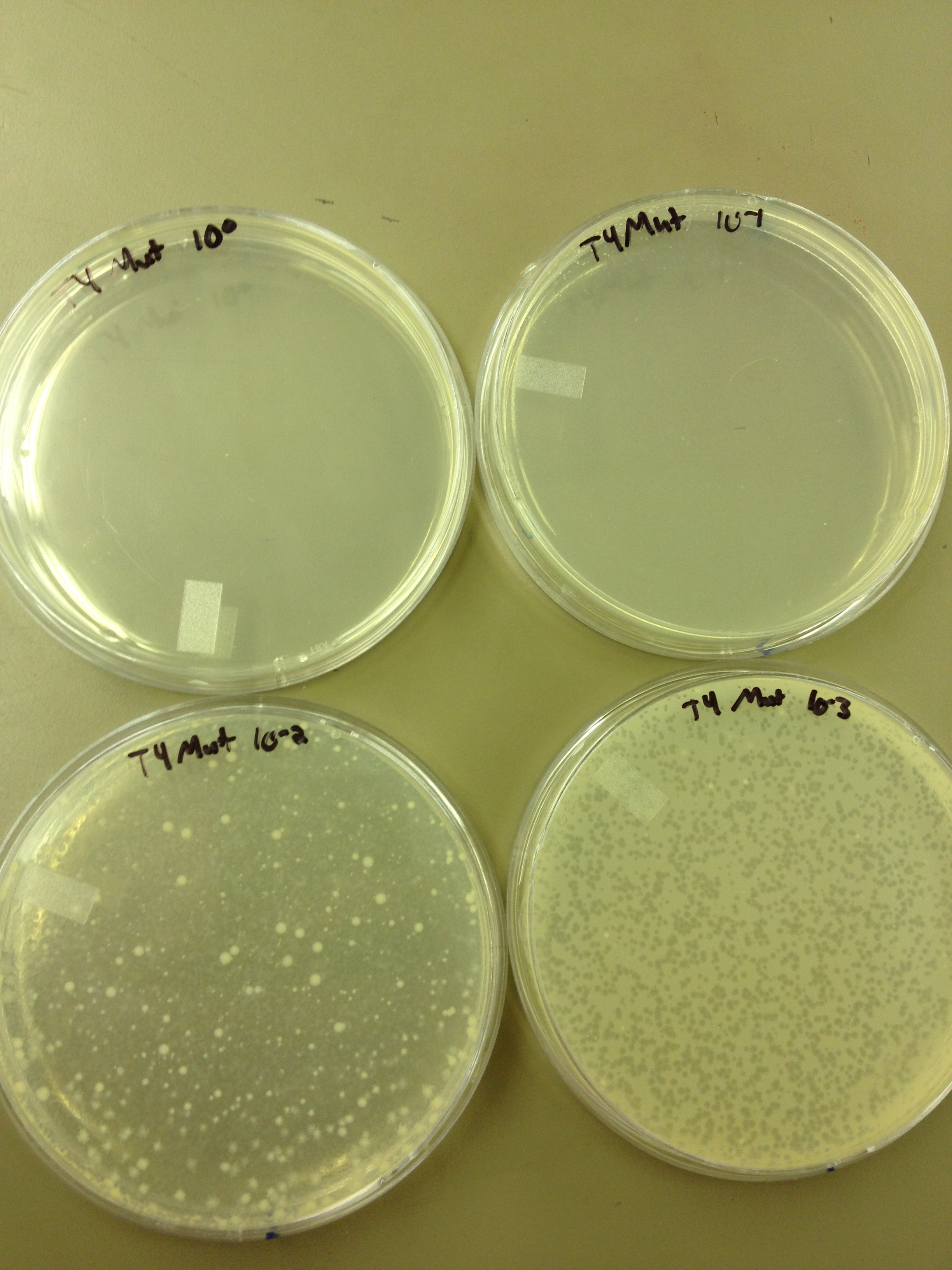
Today we did a phage titer of 0,-3,-6,-7,-8,-9,-10,-11 We started by putting 1 ul of phage into 1ml of bacteria, we went down from there. We infected each test tube with 10 uL from each dilution level.
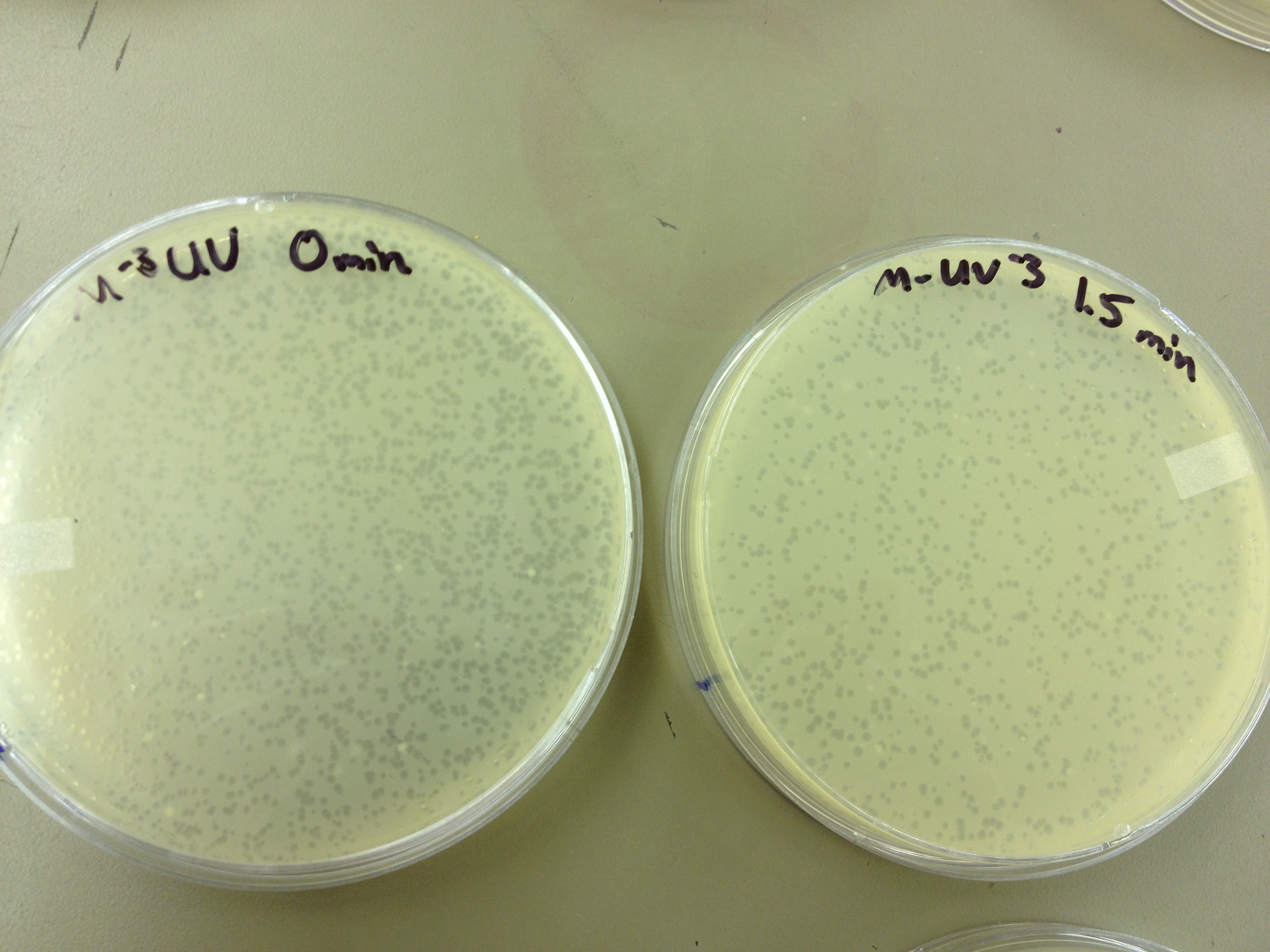
We also did a UV pre-test of sorts. We put 20ul of phage onto parafilm and exposed it to UV light in the hood of Dr. Breakwell’s lab. At 30 second intervals we took the 20 ul’s off and put it in a half ml of e.coli W3110. We tested every 30 seconds up to 3 minutes.
We also streaked out the W3110 bacteria from freezer stock onto an LB plate so we can use it to start future bacterial cultures.

We looked at the results for the dilution series (titer) we did on the phage stock we made from the liquid culture and found that there were 5 plaques on the 10^-8 plate.
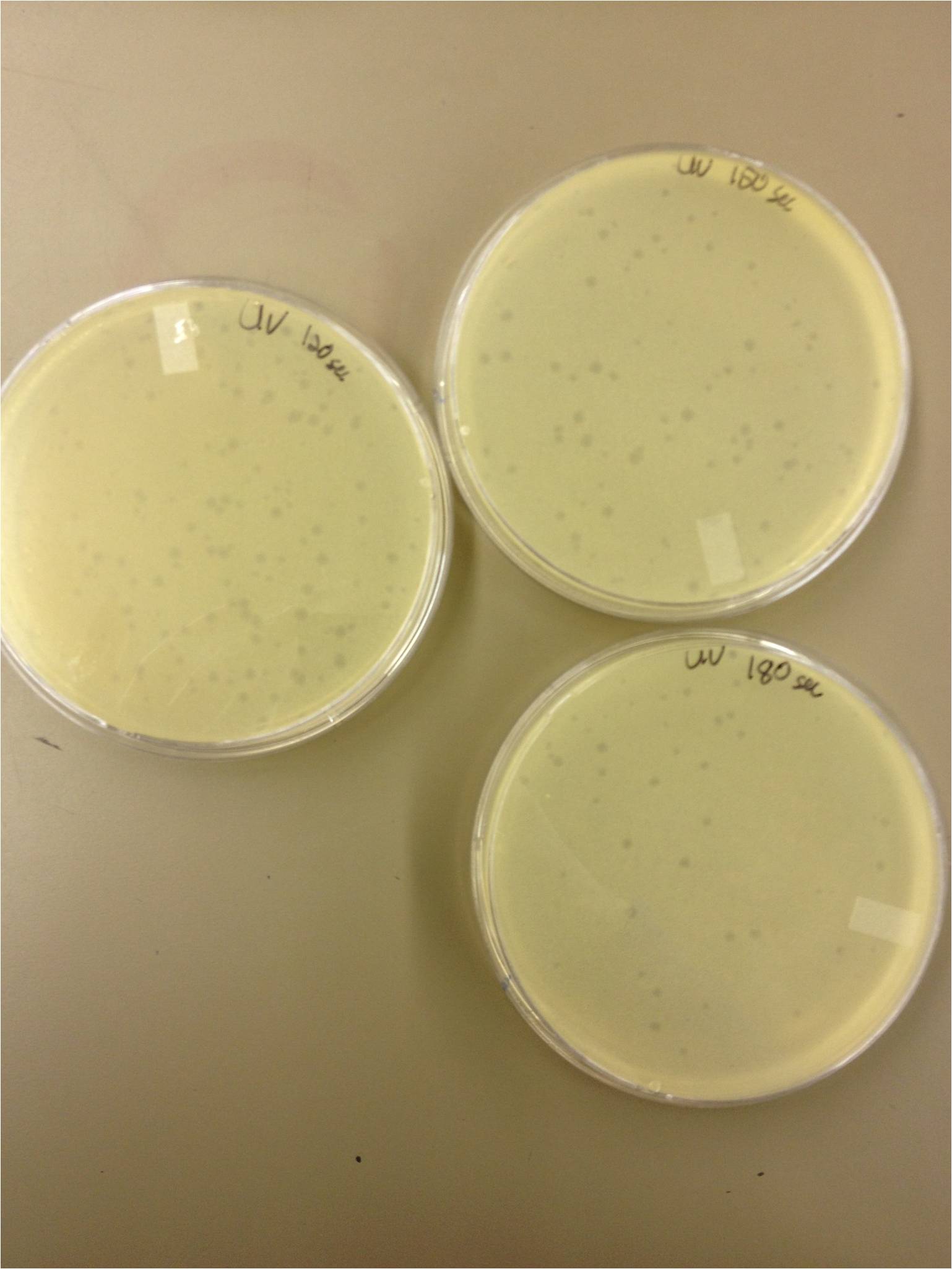
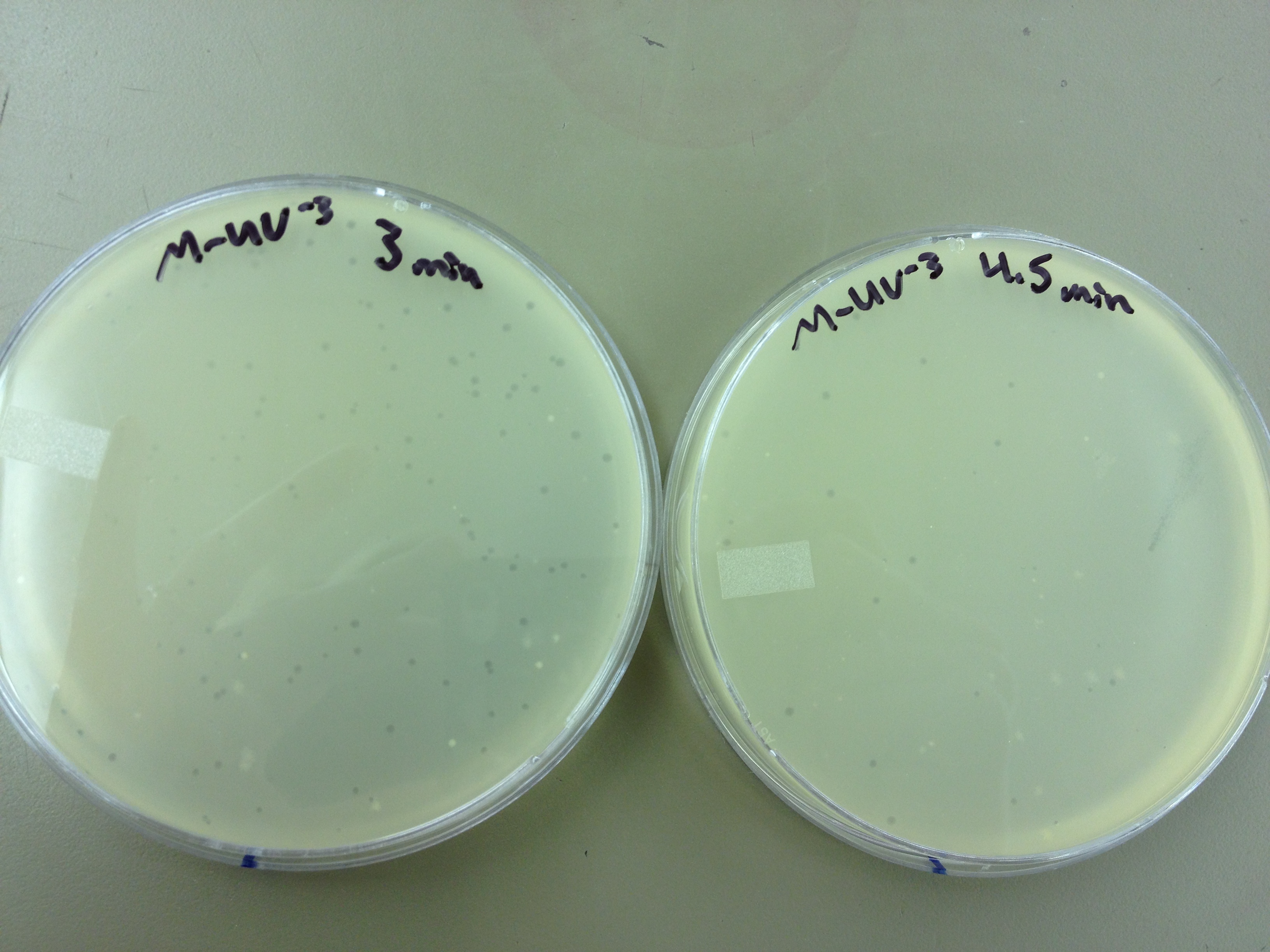
We calculated pfus/mL by doing ( # of plaques ) / (dilution level x mLs infected with). Our stock is between 3x10^9 and 5x10^9 pfus/mL. 180 sec = ~50 plaques 150 sec = ~102 plaques 120 sec = ~170 plaques 90 sec = ~280
Plaques/(dilution * amt infected with)
UV test-2
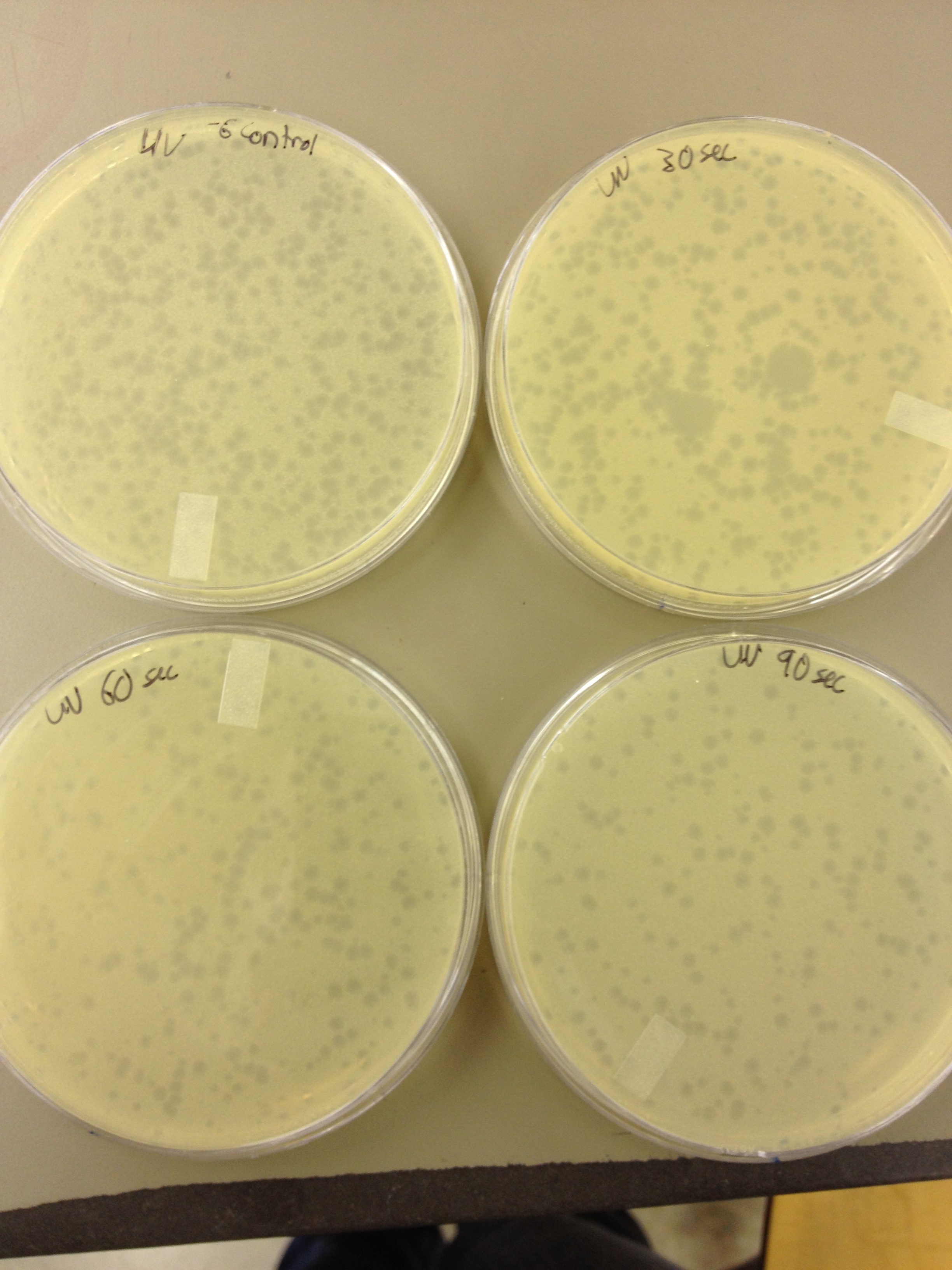
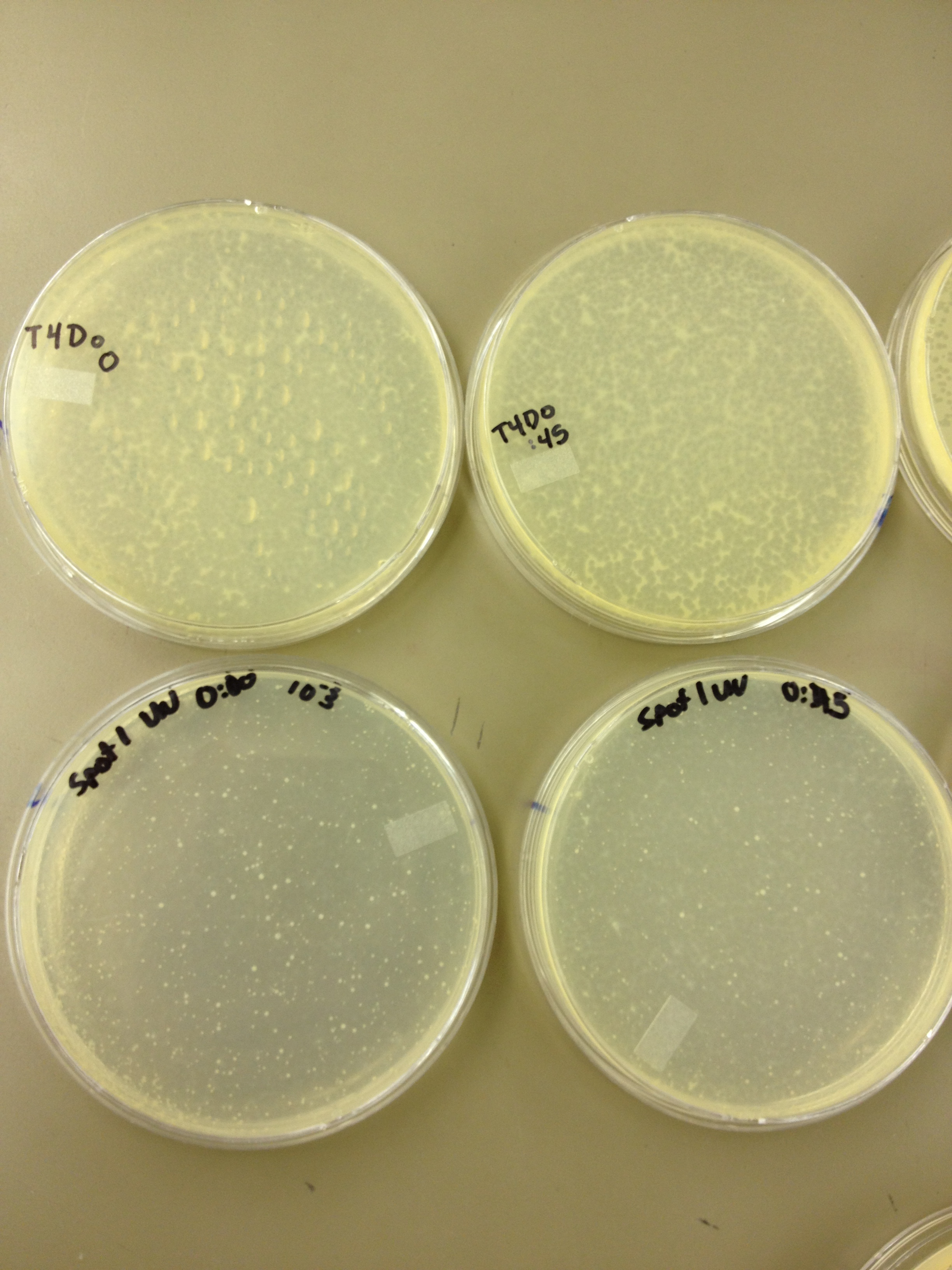
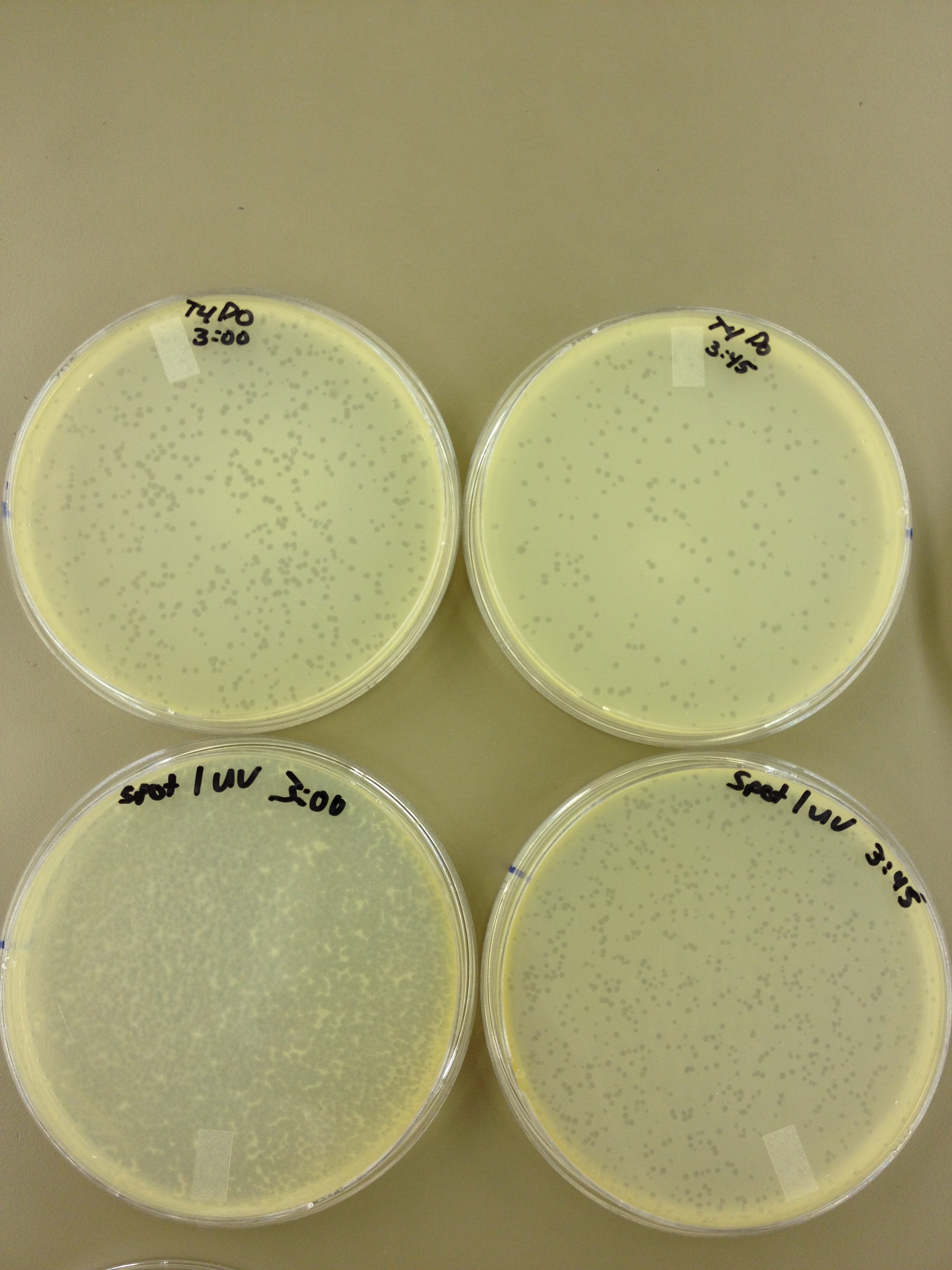
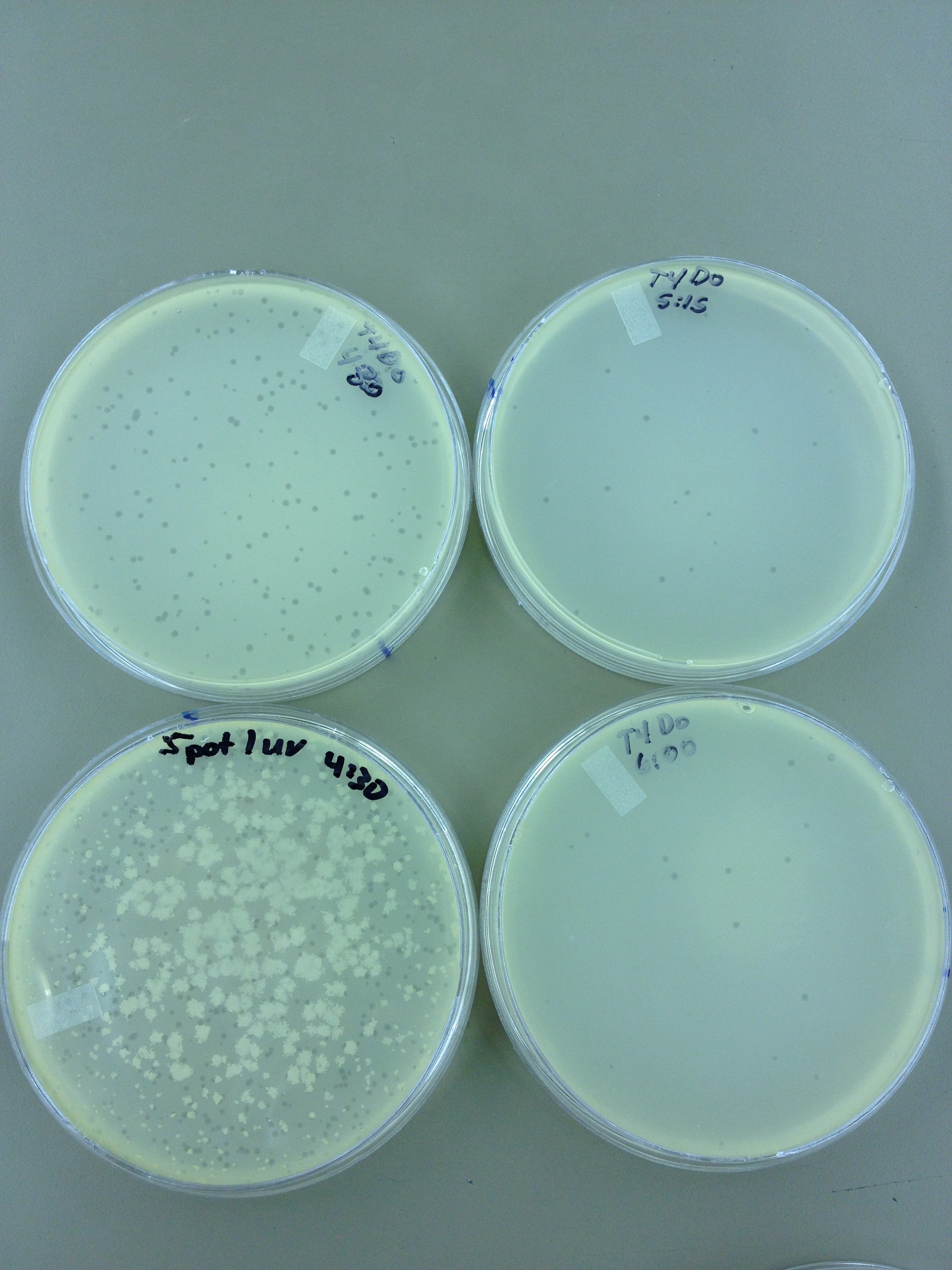
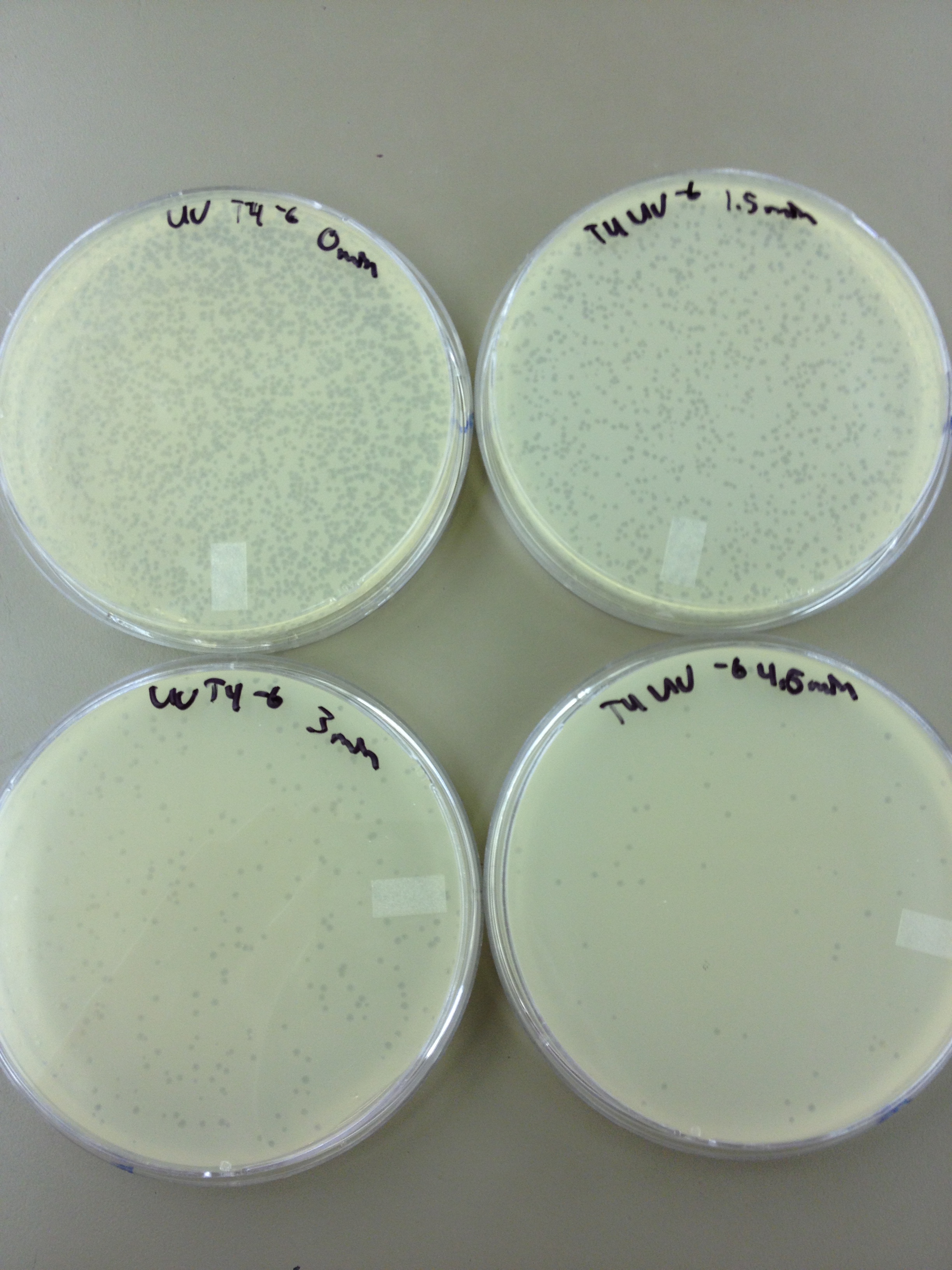
(45 Second intervals) We picked one plaque off of the 180 sec UV plate sample. We suspended it in 1 mL of broth, and then UV-ed 20 uL samples at 45 second intervals. The number of plaques decreased the longer the samples sat under UV light. The samples were irradiated from 0 sec to 4 min 30 sec.
As a control, we diluted the T4Do stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 6 min) under UV light.
Also, we diluted the T4 mutagenized stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 4 min 30 sec) under UV light.
UV tests were done by placing 20 uL spots on parafilm and placed in a BSL-2 hood with the UV light turned on.
UV test-3
Using the same procedure as for the 45 Second UV exposure set, we exposed the phage to UV in intervals of 90 seconds.
(We left the plates in the incubator for 48 hours, which caused contamination on many plates to grow.)
When checked at 24 hours, the T4 mutagenized stock had a web plate at 10^-3 and less than 5 plaques at 10^-6. This experiment will need to be redone from 10^0 down through 10^-6. The whole mutagenesis may need to be redone if this only represents a dilution of our titer when we were trying to grow it in liquid culture.
Under UV light, the T4Do stock (diluted to 10^-6) has 19 plaques after being irradiated for six minutes (down from almost cleared at 0 min).
Under UV light, the 180 sec UV plate spot (diluted in 1 mL) has a few hundred plaques on it, but the amount dropped significantly at 4 min 30 sec from when it was UV-ed for 0 min.
We will re-titer the T4-Mut stock so we can learn whether it was diluted or whether an infection worked. We will also re-run the UV test comparing the T4-Mut (10^-3) with the T4-Do stock (at 10^-6) for survivability. The mutagenized phage should survive better.
Since the loss of plaques seemed to level off for the T4Do stock at 5:15 (23 plaques) and 6 min (19 plaques), we will test a 7 min and 8 min timepoint to see if it stays level, suggesting these phage have multiple genomes and are severely mutated.
Spot Test on E.Coli B
Goals From Here
Goals for the near future: - Switch from E. coli W3110 to E. coli B (to match the paper) - Do another round of T4 mutagenesis, first supplementing with adenine - Devise a better test to confirm whether mutagenesis worked - Work further with selecting small plaques to see if they remain small - Reduce top agar concentrations to see if plaques appear that were too small for us to initially observe - Receive the files from the microscopy lab and analyze them more closely
 "
"