Monday, April 22nd
[Expand] Transformation of E. coli XL1 blue with Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Investigator: Jeff, Leonie, Rosario
Aim of the experiment: Transformation of Phytochrome B for protein fusion.
Procedure:
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 2 µl of DNA was added to 100 µl of competent cells and gently mixed.
- 5 min. heat shock at 37 °C
- Adding of 1 ml LB-medium to each tube.
- Incubation for 45 min at 37 °C in the 180 rpm cell-culture shaker.
- 100 µl of the cell suspension was plated on one chloramphenicol plate.
- The rest were centrifuged for 1 min at 13000 rpm and the supernatant was dicarded.
- The pellet was resuspended in 100 µl of LB-medium and this concentrated cell suspension was plated again on a new chlorampenicol plate.
[Expand] Miniprep of pTUM100 with pGAL, pTEF1, pTEF2, pADH and RFC25 compatible RFP generator
Investigator: Jeff, Leonie, Rosario
Aim of the experiment: Miniprep of pTUM100 with pGAL, pTEF1, pTEF2, pADH and RFC25 compatible RFP generator
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
[Expand] Sequencing of RFP-Generator (RFC25, pSB1C3)
Investigator: Jeff, Leonie, Rosario
Aim of the experiment: Sequencing of RFP-Generator (RFC25, pSB1C3)
Procedure:
Sequencing batch were prepared after manufacturer's protocol. (15 µl of plasmid DNA (50 - 100 ng) and 2 µl sequencing primer)
Tuesday, April 23rd
[Expand] Picking of of E. coli XL1 blue with Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Investigator: Jeff, Leonie, Rosario, Florian
Aim of the experiment: Picking of of E. coli XL1 blue with Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Procedure:
- pSB1C3 plasmid with BBa_K801031 (PhyB 2 - 908 aa, RFC25): Colonies were picked from chloramphenicol plates.
- Picked pipette tips was transferred into cell-culture tubes with air-permeable, sterile cover. Each tube contain 4 mL of LB-medium + 4 µL chloramphenicol(1000x).
- These tubes were transferred in a cell culture shaker at 37 °C and were incubated overnight
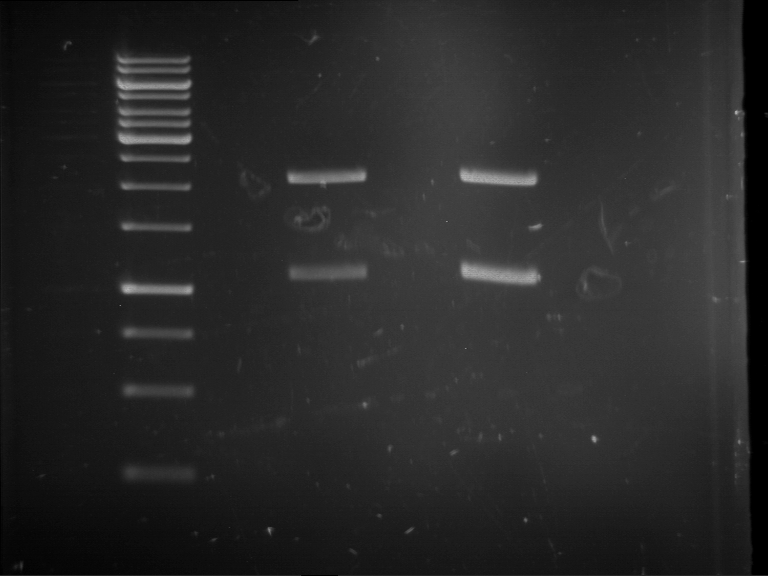
[Expand] Analytical digestion and gelelectrophoresis of RFP-generator (RFC25, pSB1C3, P4 & P5)
Investigator: Jeff, Leonie, Rosario, Florian
Aim of the experiment: Analytical digestion and gelelectrophoresis of RFP-generator (RFC25, pSB1C3, P4 & P5).
Procedure:
- Batch for analytical digestion for P4 with NgoMIV+AgeI-HF
| volume
| reagent
|
| 2.5 µl
| Plasmid DNA P4
|
| 2 µl
| NEBuffer 4 (10x)
|
| 0.25 µl
| NgoMIV (10 U/µl)
|
| 0.25 µl
| AgeI-HF (20 U/µl)
|
| 15 µl
| ddH2O
|
| =20 µl
| TOTAL
|
- Batch for analytical digestion for P5 with NgoMIV+AgeI-HF
| volume
| reagent
|
| 2.5 µl
| Plasmid DNA P5
|
| 2 µl
| NEBuffer 4 (10x)
|
| 0.25 µl
| NgoMIV (10 U/µl)
|
| 0.25 µl
| AgeI-HF (20 U/µl)
|
| 15 µl
| ddH2O
|
| =20 µl
| TOTAL
|
- Incubation for 90 min at 37 °C.
- Analytical gelelectrophoresis was performed at 90 V for 60 min.
Results:
| 1 kbp ladder DNA ladder
| P4
| P5
|
|
| Mutation successful
| Mutation successful!
|
- Parts are compliant and do not contain RFC25 forbidden restriction sites.
[Expand] Sequencing of pTUM vectors with pGAL, pADH, pTEF1, pTEF2
Investigator: Jeff, Leonie, Rosario, Florian
Aim of the experiment: Sequencing of pTUM vectors with pGAL, pADH, pTEF1, pTEF2
Procedure:
Sequencing batch were prepared after manufacturer's protocol. (15 µl of plasmid DNA (50 - 100 ng) and 2 µl sequencing primer).
The different vectors we sequenced received the following barcodes:
- ADH in pTUM100: FR01002265
- TEF1 in pTUM100: FR01002266
- TEF2 in pTUM100: FR01002266
- GAL in pTUM100: FR01002268
Sequencing of TEF2 in pTUM100 was not interpretable. The other sequences were consistent with the sequences in the parts registry.
Wednesday, April 24th
[Expand] Miniprep of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Investigator: Jeff, Leonie, Florian
Aim of the experiment: Miniprep of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3).
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
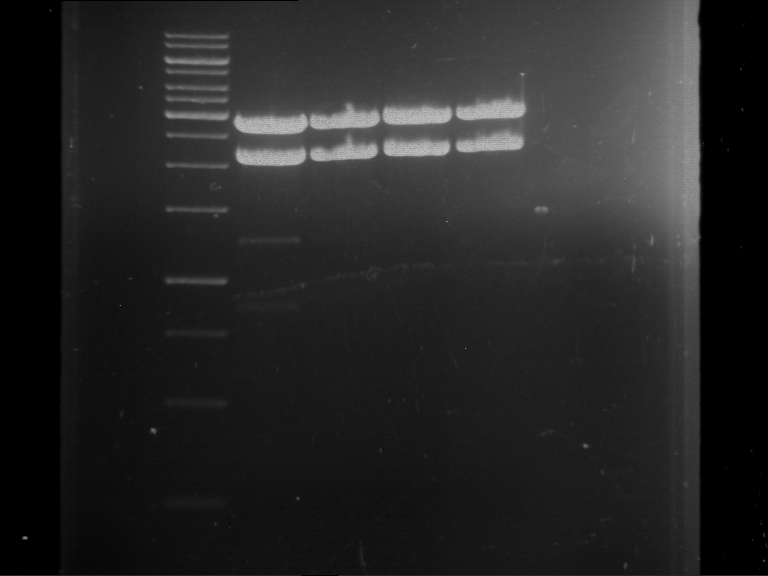
[Expand] Analytical digestion and gelelectrophoresis of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3), P7 - P10
Investigator: Jeff, Leonie, Florian
Aim of the experiment: Analytical digestion and gelelectrophoresis of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3), P7 - P10.
Procedure:
- Batch for analytical digestion for P7 with NgoMIV+AgeI-HF
| volume
| reagent
|
| 2.5 µl
| Plasmid DNA P7
|
| 2 µl
| NEBuffer 4 (10x)
|
| 0.25 µl
| NgoMIV (10 U/µl)
|
| 0.25 µl
| AgeI-HF (20 U/µl)
|
| 15 µl
| ddH2O
|
| =20 µl
| TOTAL
|
- Batch for analytical digestion for P8 with NgoMIV+AgeI-HF
| volume
| reagent
|
| 2.5 µl
| Plasmid DNA P8
|
| 2 µl
| NEBuffer 4 (10x)
|
| 0.25 µl
| NgoMIV (10 U/µl)
|
| 0.25 µl
| AgeI-HF (20 U/µl)
|
| 15 µl
| ddH2O
|
| =20 µl
| TOTAL
|
- Batch for analytical digestion for P9 with NgoMIV+AgeI-HF
| volume
| reagent
|
| 2.5 µl
| Plasmid DNA P9
|
| 2 µl
| NEBuffer 4 (10x)
|
| 0.25 µl
| NgoMIV (10 U/µl)
|
| 0.25 µl
| AgeI-HF (20 U/µl)
|
| 15 µl
| ddH2O
|
| =20 µl
| TOTAL
|
- Batch for analytical digestion for P10 with NgoMIV+AgeI-HF
| volume
| reagent
|
| 2.5 µl
| Plasmid DNA P10
|
| 2 µl
| NEBuffer 4 (10x)
|
| 0.25 µl
| NgoMIV (10 U/µl)
|
| 0.25 µl
| AgeI-HF (20 U/µl)
|
| 15 µl
| ddH2O
|
| =20 µl
| TOTAL
|
- Incubation for 90 min at 37 °C.
- Analytical gelelectrophoresis was performed at 90 V for 60 min.
Results:
| 1 kbp ladder DNA ladder
| P7
| P8
| P9
| P10
|
|
| Part is correct
| Part is correct
| Part is correct
| Part is correct
|
[Expand] Transformation of E. coli XL1 blue with EreA and EreB (pDEST14)
Investigator: Jeff, Leonie, Florian
Aim of the experiment: Transformation of E. coli XL1 blue with EreA and EreB (pDEST14).
Procedure:
- Plasmid DNA was received dried in paper from McMaster University.
- DNA was resuspended in ddH2O
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 2 µl of DNA was added to 100 µl of competent cells and gently mixed.
- 5 min. heat shock at 37 °C
- Adding of 1 ml LB-medium to each tube.
- Incubation for 45 min at 37 °C in the 180 rpm cell-culture shaker.
- The transformated cells were centrifuged for 1 min at 13000 rpm and the supernatant was dicarded.
- The pellet was resuspended in 100 µl of LB-medium and this concentrated cell suspension was plated on chlorampenicol and ampicillin plates.
Monday, April 29nd
[Expand] Miniprep of pRK792, pRK793, pRIL, ereA, ereB
Investigator: Leonie
Aim of the experiment: Miniprep of pRK792, pRK793, pRIL, ereA, ereB
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
- Concentrations were determined by measuring the absorption:
| Plasmid
| c [ng/µl]
|
| pRK792
| 214,5
|
| pRK793
| 154,3
|
| pRIL
| 6,1
|
| ereA
| 183,4
|
| ereB
| 98,3
|
- The procedure will have to be repeated for pRIL
[Expand] Midiprep of RFC25 compatible RFP generator in (pSB1C3)
Investigator: Andreas, Florian
Aim of the experiment: Midiprep of RFC25 compatible RFP generator in (pSB1C3)
Procedure:
- Midiprep was performed after manufacturer's protocol (QIAprep Midiprep, QIAGEN)
 "
"






