|
- Overview
- March-April
- May-June
- July-August
- September-October
|
4.25 T7 phage viability test
I) Purpose
- Test the viability of the E coli BL21 and T7+
- Perform T7+ titer to determine a workable concentration for selection test
- Test phage viability in liquid culture
II) Expected Outcome
- E coli BL21 overnight liquid culture should show normal growth; streaking should give multiple colonies
- T7 spot test should give results similar to that seen in 4.8 and 4.10 phage viability assay
- T7 titer should give results similar to that seen in 4.10 phage viability assay
- Phage liquid culture should see clearage, but not in control liquid culture
III) Reagants Used
- T7 + phage: dilution from 4.8; E coli BL21; Agar: ×2 prepared by Jordan in 500mL glass bottle; LB: prepared by us on 4.3; overnight bacteria culture: set up on Thursday at 11:15pm using 4.6 streak
IV) Actual Procedure
1. Streak test for E coli BL21
- i) E coli overnight liquid culture was set up the night before. At 3.25 10:30 am obvious signs of bacterial growth were seen. Streak test was then performed to check bacteria viability and prepare for storage.
- ii) As of 5:00pm NO contamination was seen on plate. We thus proceed to the next step.
2. Perform spot test
- i) 0.5mL E coli overnight + 5mL ×1 top agar -> two plates: one for control, one for actual spot test.
- ii) Divide spot test plate into 6 sections, labeled -4 through -9
- iii) Spot 5 μL of phage solution onto each of the sections. Concentration of phage should match the labeled number.
- iv)Both plates were incubated right side up.
3. Perform titer test
- i) Four test tubes were labeled -4 through -7. To each of them, 0.5mL of E coli and 20 μL of phage solution (at concentration matching the labeled number) were added. Let this mixture sit for 20 minutes
- ii) To each test tube add 5mL of ×1 top agar. Plate.
- Note: -7 did not solidify after plating and was disposed before incubation.
- iii) Incubate up side down (4.25 6:00pm – 4.26 9:30am for 15.5hr)
4. Liquid culture test
- i) 2.mL LB + ½ colony of E coli BL21 was added to two test tubes
- Note: colony size is based on estimation, this introduces a huge variable into the experiment.
- ii) To one of the test tubes add an extra 20 μL of phage solution (-5); the other will serve as control
- iii) Incubate for almost two days (4.25 6:00pm – 4.27 2:00pm)
- iv) The supernatant was collected, transferred to two 1.5mL centrifuge tubes, and stored in the fridge.
V) Results
1. Plates
Plate Results
| Plate
| Results
|
| Streak test
| Multiple colonies; no infection; too many colonies and too small to be stored
|
| Spot test - control
| Uniform lawn of E coli, no contamination seen
|
| Spot test – experiment (-4 through -9)
| Obvious clearage for -4 through -6; -7 has a pin-size prick
|
| Titer test -4
| Overlapping plaques
|
| Titer test -5
| Individual plaques that overlaps in some places
|
| Titer test -6
| Individual plaques; streaking seen, possibly due to incubating before agar has completely solidified
|
| Titer test -7
| Did not solidify and was disposed before incubation
|
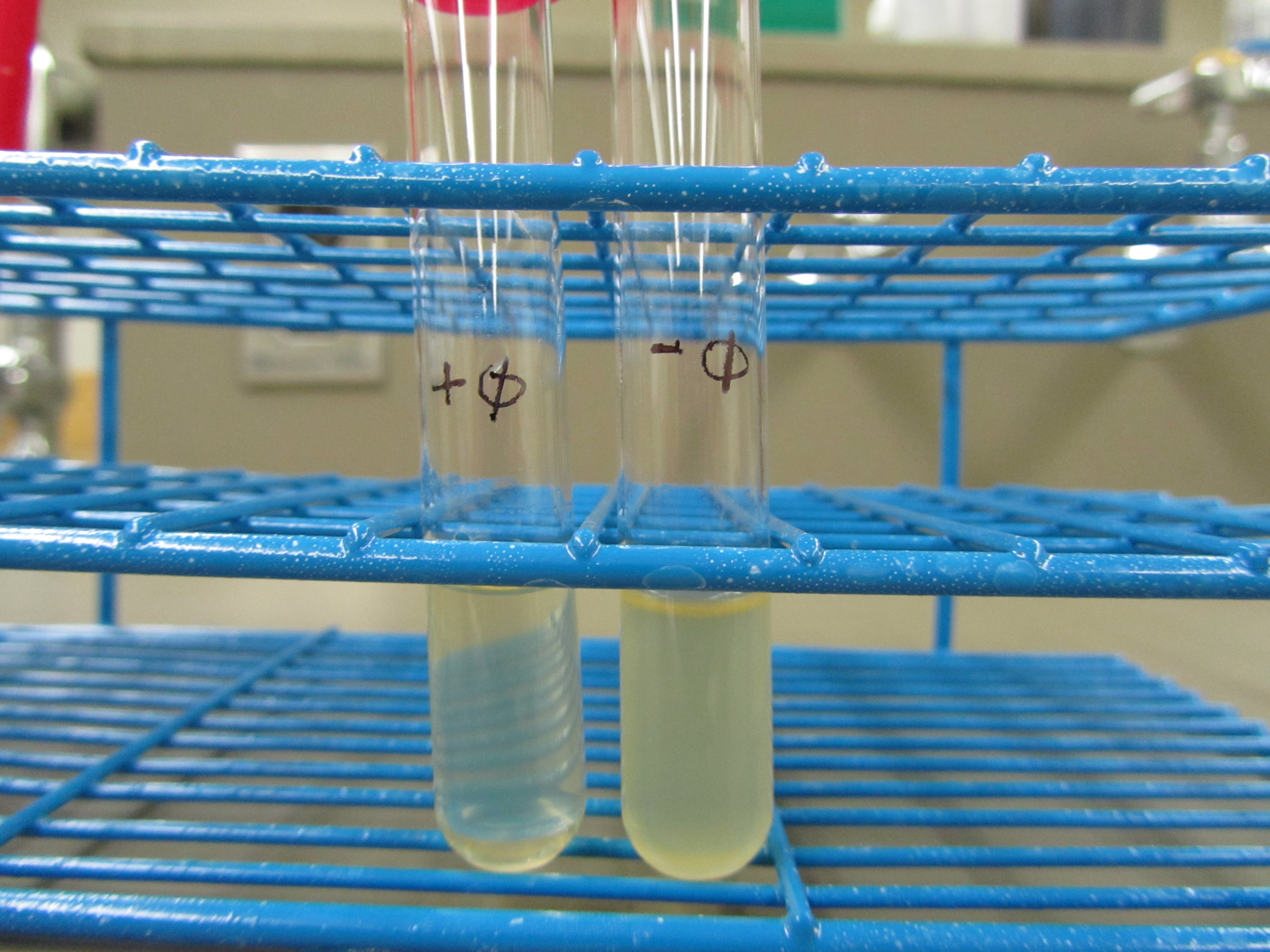
| Liquid culture test
| As compared to the control, + phage is much more clear
|
2. Spot test results
This is similar to what we saw before finals. This confirms that our phage dilution series have survived. Hurray!
3. Titer test result
-5 and -6 will be a good place to start our selection test. We will also need to increase phage titer (phage concentration).
4. Liquid culture test (4.27 6:00pm)
Obvious clearage is seen in + phage test tube. E coli debris was also observed at the bottom of the + phage test tube.
VI) Conclusion
- T7+ have survived the final. We can continue to use the 4.8 dilution series for our experiments. This will save us some stock phage.
- -5 is a good phage concentration to start our selection test.
- The stock phage solution has a medium titer, we need to increase it to perform our mutation experiments.
VI) Proposed next step
- Perform selection test with -5 phage.
- Increase titer of stock phage for mutation experiments.
|
 "
"