|
- Results Overview
- Judging Criteria
- * Bronze
- * Silver
- * Gold
- Experimental Results
- Modeling Results
- Parts Submitted
|
Phage Team
Isolation of Phage Library
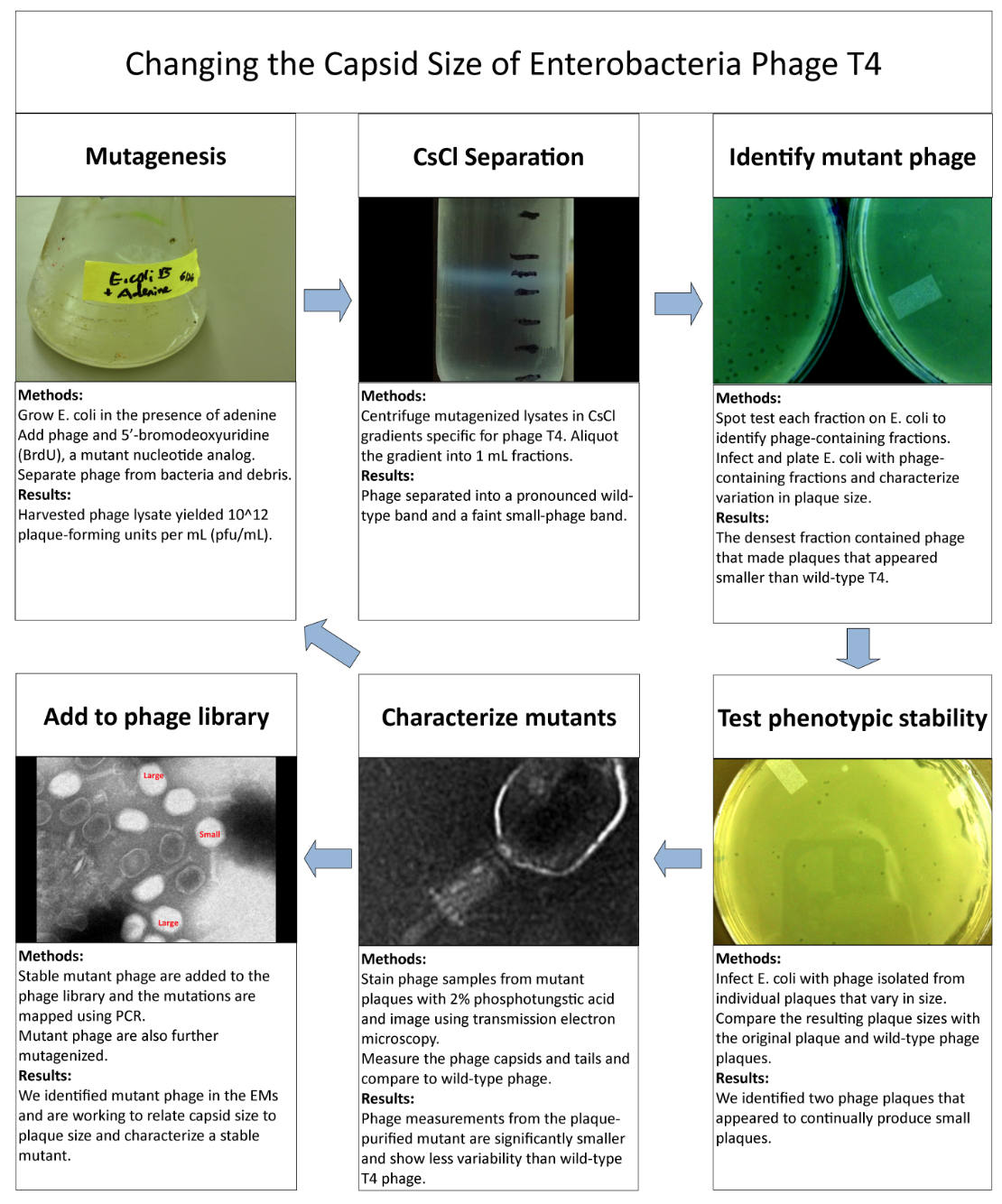
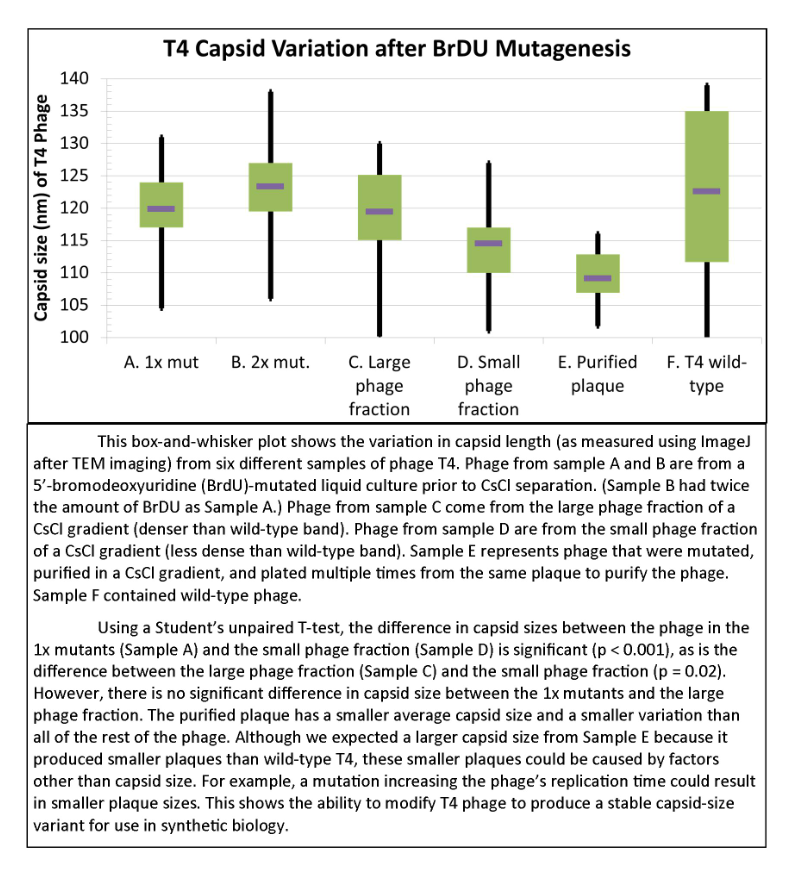
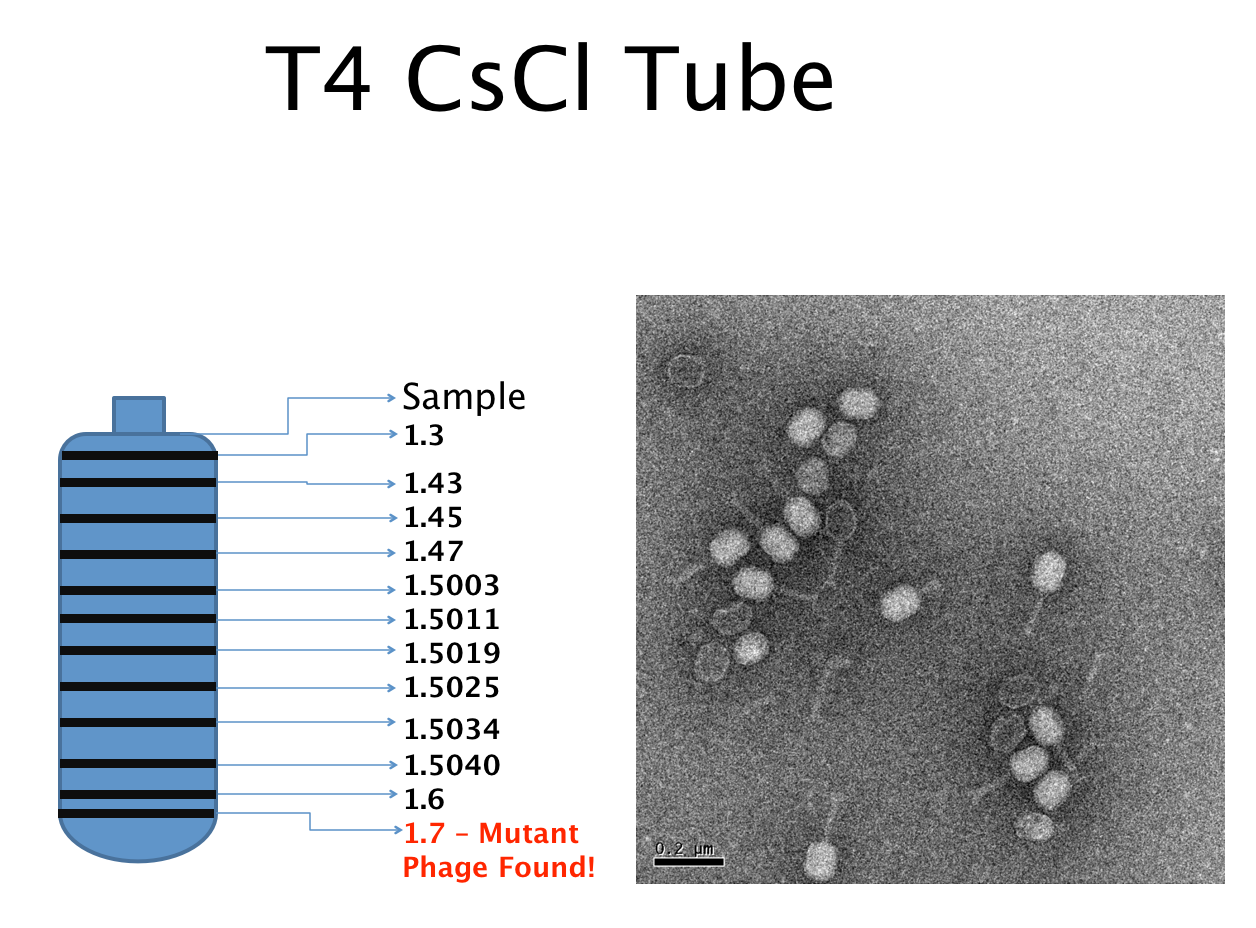
Results of Mutant T4 Phage Isolation
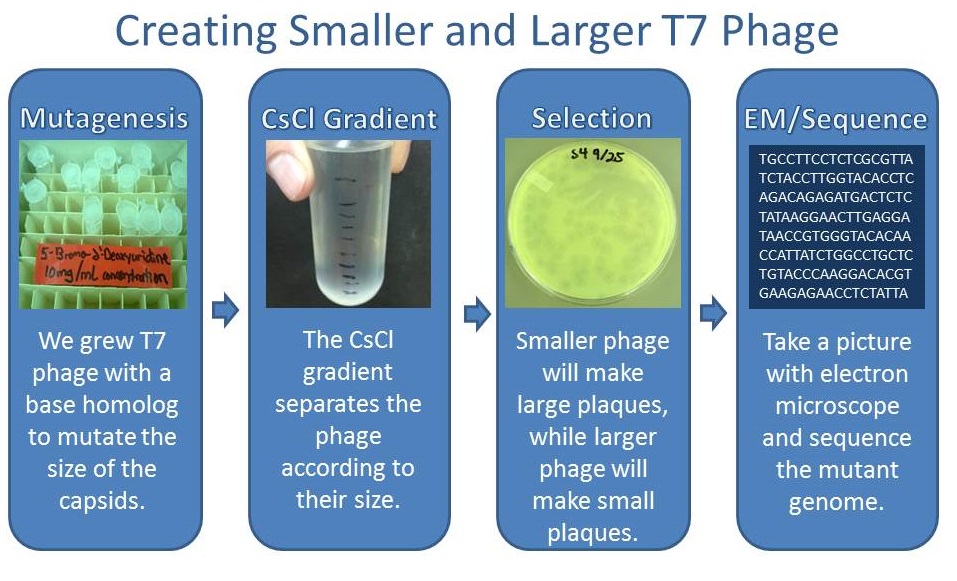
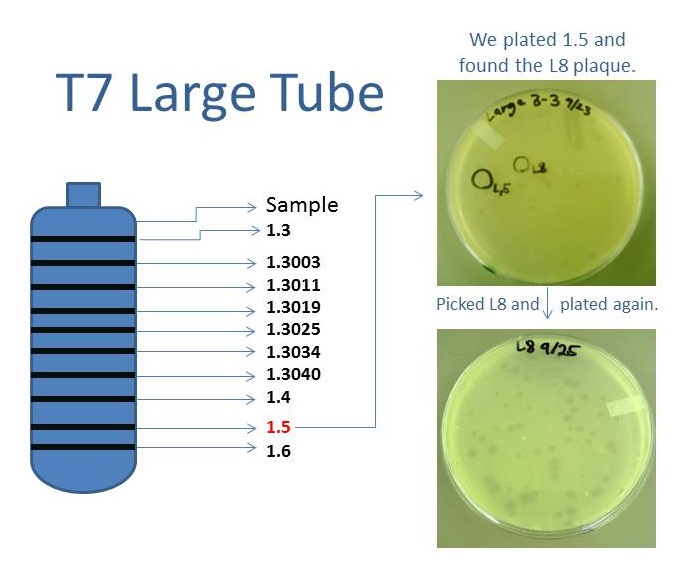
Results of Mutant T7 Phage Isolation
As part of creating our bacteriophage library, we wanted to isolate both smaller and larger T7 bacteriophage. To do so, we first mutagenized T7 by growing it in the presence of 5-bromodeoxyuridine, a mutagenic base analog. This produces many mutant T7 bacteriophages, but they are hard to find amongst all the wild type T7. Therefore the phage purification team ran the mutagenized bacteriophage through a cesium chloride gradient. This separates the phage according to size, with the biggest traveling farther down. After determining where the mutant phage is in the gradient, we then plated the phage and looked for plaques that were smaller or larger than normal. Out of the 31 plaques we selected, two of them reproduced their plaque sizes, revealing that they were indeed mutant T7 bacteriophage! The next step will be to get pictures of the phage with an electron microscope and sequence the genome to determine where the mutations occurred.
Cholera Team
Cholera Induces Bacteriophage Lambda From Lysogeny to its Lytic Cycle
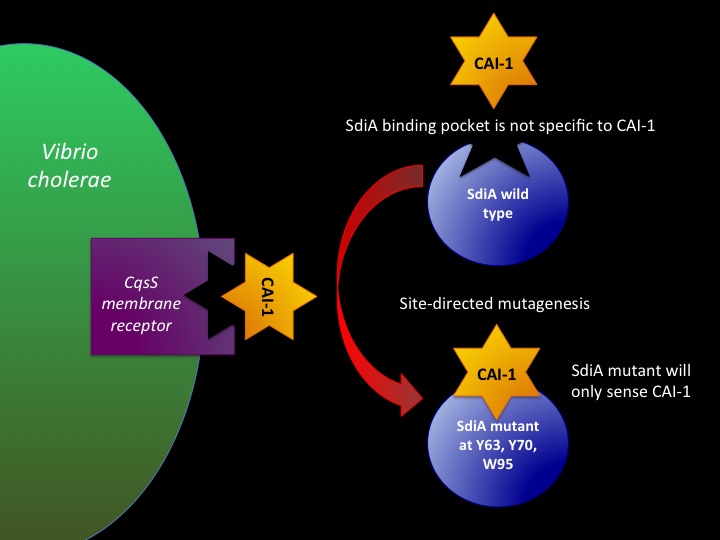
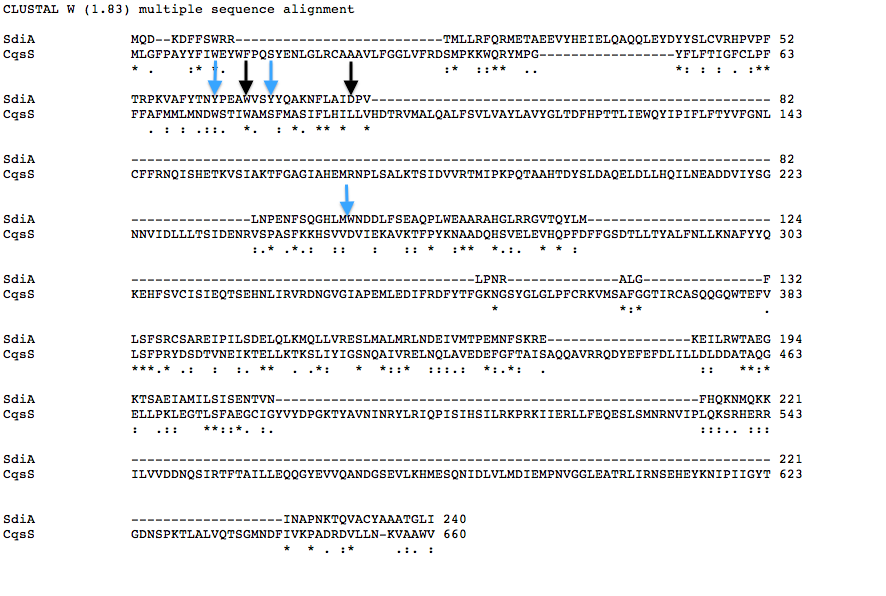
Designing SdiA to be Specific for Cholera
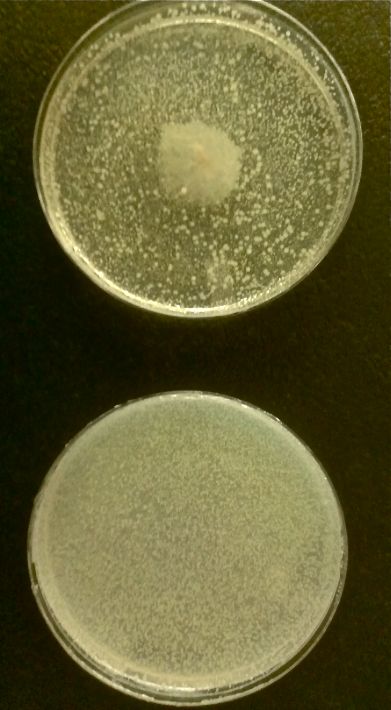
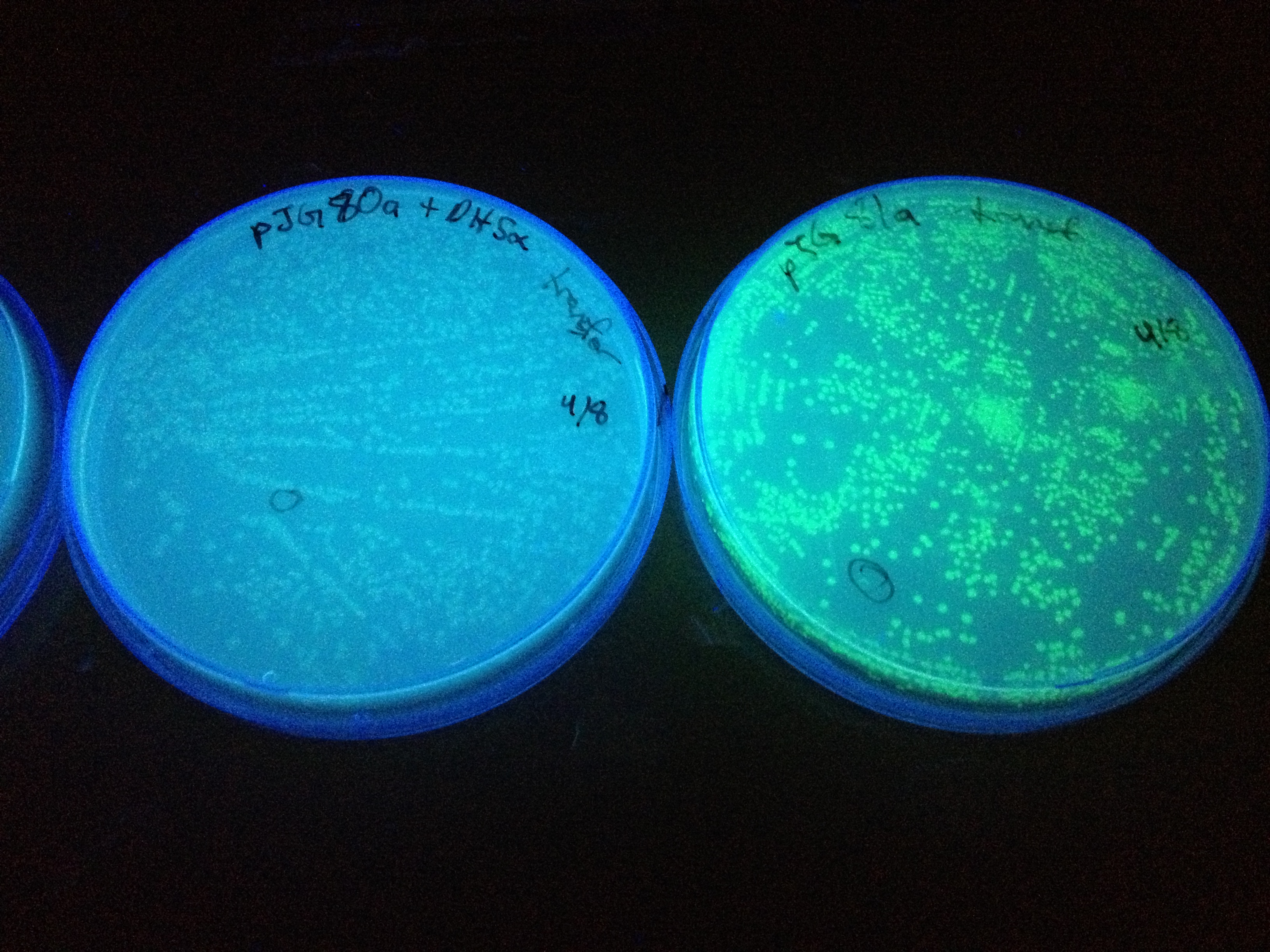
In the beginning our plan was to transform into E.coli the most essential parts of the V.cholerae quorum sensing system. This was done, but our response proteins GFP and RFP did not work as expected.Shown above are colonies that were not supposed to be glowing. So we decided to try mutating the quorum sensing system E.coli already had to be able to only sense CAI-1 which is an auto-inducer specific to V.cholerae.
Biofilm inhibition by Amylase
The enzymatic activity of α-Amylase was characterized to determine its capacity to inhibit biofilm formation by V. cholerae. Samples were prepared by adding 50 µL of V. cholerae culture to 1 mL of a high salt LB. The samples were then treated by the addition of purified α-Amylase in various concentrations and allowed to incubate at 30°C for 48 hours. After 48 hours the samples were examined and a distinct difference was seen in the amount of biofilm formed in the treated and untreated samples.
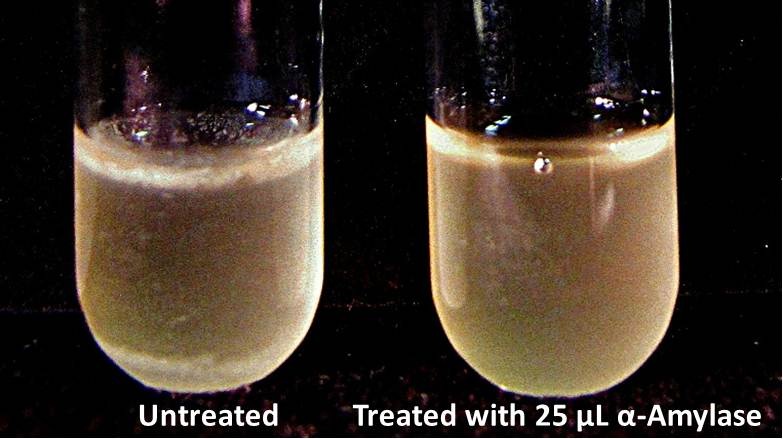 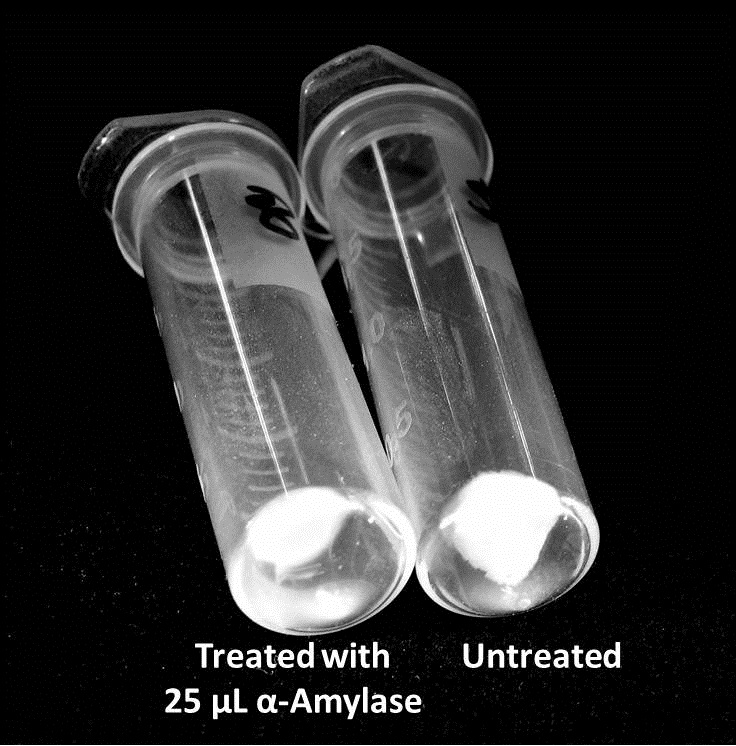
The samples were then transferred to eppendorf tubes and centrifuged at 16,000 × g for two minutes, the supernatant was discarded to remove the growth media, and the samples were resuspended in 200 µL ddH2O. The samples were then stained with 50 µL of a 0.03% CV solution and allowed to incubate for five minutes. The samples were again centrifuged at 16,000 × g for two minutes and the supernatant containing excess CV was discarded. The pelleted biofilm was then washed with 800 µL of 95% EtOH without resuspension, centrifuged for 30 seconds, and the EtOH was discarded. The EtOH wash was repeated twice more for a total of three washes. The samples were then resuspended in 200 µL EtOH, transferred to a 96-well plate, and incubated for five minutes. The plate was then shaken for ten seconds and absorbance readings were taken for each sample at 540 nm, the absorbance wavelength of CV.
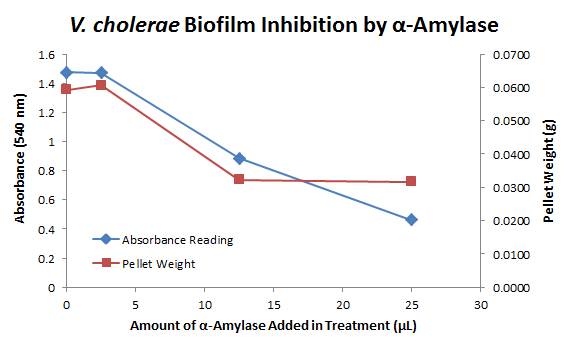 The above graph shows both the average pellet weight and the average absorbance readings for samples treated with 0, 2.5, 12.5, and 25 µL of α-Amylase. There is a distinct reduction in the amount of biofilm growth between untreated and treated samples, with samples treated with 25 µL α-Amylase showing a 65.8% decrease in biofilm formation after 48 hours. While this clearly shows the ability of α-Amylase to inhibit biofilm formation by V. cholerae, further characterization is needed to determine the capacity of α-Amylase to degrade preexisting biofilms.
|
 "
"