Team:KU Leuven/Journal
From 2013.igem.org
Secret garden
Congratulations! You've found our secret garden! Follow the instructions below and win a great prize at the World jamboree!
- A video shows that two of our team members are having great fun at our favourite company. Do you know the name of the second member that appears in the video?
- For one of our models we had to do very extensive computations. To prevent our own computers from overheating and to keep the temperature in our iGEM room at a normal level, we used a supercomputer. Which centre maintains this supercomputer? (Dutch abbreviation)
- We organised a symposium with a debate, some seminars and 2 iGEM project presentations. An iGEM team came all the way from the Netherlands to present their project. What is the name of their city?
Now put all of these in this URL:https://2013.igem.org/Team:KU_Leuven/(firstname)(abbreviation)(city), (loose the brackets and put everything in lowercase) and follow the very last instruction to get your special jamboree prize!


Team
11/07
We decided to take advantage of the nice weather and had a BBQ. The morning after the iGEM-room was pretty empty, which might had something to do with the beers...
14/07
Members of this and previous years' Calgary iGEM team came to visit Leuven and, after a few belgian bears, were willing to give us lots of tips & tricks to get through iGEM summer.
Modeling
01/07
We installed Matlab, took a look at some presentations about modeling. We are also investigating [http://www.mathworks.nl/products/simbiology/ SimBiology].
02/07
We watched some [http://www.mathworks.nl/products/simbiology/webinars.html webinars about SimBiology]. In the afternoon we had an appointment with professor Bernaerts of the division of (bio)chemical procestechnology. It was very useful, as she gave some good ideas on how to get started. We will have to design a way of expressing the enzyme(s) in a cyclic manner. We could achieve this by expressing it during a short pulse, activated by the presence of a signal from other cells.
03/07
During the morning we brainstormed about some possible networks with oscillating behavior. We have to keep in mind that the colony has to be and stay synchronized. This could be achieved by a rapid (protein-protein interaction) feedback which is proportional to the phase difference.
We had a meeting with the wetlab team and discussed our main focus for the upcoming weeks: figuring out an oscillating construct and simulating the behaviour of the methyl salicylate BioBrick.
04/07
Bert and Sander are working on simulating the ″Mortier Oscillator″ in SimBiology.
Tina and Tomas are modeling the network to produce methyl salicylate.
- pchA and pchB catalyze the reactions from chorismate to isochorismate to salicylate.
- BSMT1 is the enzyme that catalyzes the reaction from salicylate to methyl salicylate.
Tomas and Sander are looking on how to use the [http://opencobra.sourceforge.net/openCOBRA/Welcome.html COBRA toolbox] for our purpose. We would need to check the constraints on reaction rates and add our new reactions. Bert made contact with professors Suykens of the [http://www.esat.kuleuven.be/scd/ department of electrical engineering] and professor Degrève of the division of (bio)chemical procestechnology in order to have an idea of how to analyse the MO, we apparently need [http://en.wikipedia.org/wiki/Bifurcation_theory bifurcation analysis], which Bert started looking up about.
05/07
Tomas started the development of another oscillator, while Sander and Tina are looking deeper into metabolic network modeling.
07/07
We are in touch with professor Roose of the [http://wms.cs.kuleuven.be/groups/natw/index.html applied mathematics division], who suggested the use of [http://www.matcont.ugent.be/ MatCont] for the analysis of our oscillator.
08/07
We’re investigating the [http://pubs.acs.org/doi/abs/10.1021/sb300084h AutoBioCAD] software and looking for a better model to our quorum sensing system using PDEs.
09/07
We started investigation of literature of ecology. We chose our model species (aphid and crop). We’ll try to model aphid reproduction and the influence of bèta-farnesene and methylsalicylate on life cycle and movement. We’ll have to make an estimation of the damage/loss of crops due to a certain population of aphids. Bert added spatial heterogeneity in his Mortier Oscillator to see what happens on a population level.
10/07
We had a meeting Jonas Demeulemeester, member of the KULeuven iGEM team of 2008, who explained us the methods their modeling team used. It was a lot of help to us and now we are at a new start!
11/07
Today was a Flemish holiday and we couldn't make use of our HQ, so we decided to work at Agora, the KU Leuven study centre. Tomas was finally able to solve the 2D PDE in Matlab.
12/07
Tomas refined the PDE and boundary conditions for the diffusion. A more realistic model for the wind speed was used.
Wetlab
26/06
Some of us have already finished their exams so we started working in the lab. For now we just made the antibiotics solutions that we will have to use this summer.
27/06
Today we poured the agar plates with and without the antibiotics.
28/06
Just one thing to do today: make the FSB buffer to make competent cells. Easy job, but we have to recover from the exams, and it is almost weekend.
01/07
As the first day of July, our job today wasn't too heavy. We started making the competent cells. The main task was to inoculate Top10 and DH5alpha strains from agar plate into the medium, herein we use tips to transfer both the strains into 3ml LB medium under laminar flow, two times for each strain. Then incubate them overnight.
02/07
In the morning we continued yesterday's job, working with competent cells and in the afternoon we tried the transformation efficiency kit. Fingers crossed!
03/07
We checked the plates that grew under 37°C overnight, and surprisingly no colony appear in any of them! Now we have to figure out what went wrong. Possible reasons are:
- Bad competent cells (please not!)
- We didn't use enough recovery time for the cells.
- The cells need more time to grow.
To examine what went wrong we did the experiment over again, but this time we used our own pUC 19-vector and let the cells recover for 2 hours instead of one.
We also prepared the SOC-medium for future use.
04/07
Hurray! This time we do have cell growth, so we counted the cells and calculated the transform efficiency. This was still quite low though... We prepared the GTE-buffer for future use and prepared agar medium. In the afternoon, we found out that the medium in the autoclave spilled out everywhere in the autoclave, possibly due to the pressure in the autoclave. Lukas was the lucky guy who got to clean out the autoclave.
05/07
We selected 16 parts to work with further
| Plate 1 | Plate 2 | Plate 3 | Plate 4 | Plate 5 |
|---|---|---|---|---|
| 1C [http://parts.igem.org/Part:BBa_K398326 BBa_K398326] | 1I [http://parts.igem.org/Part:BBa_B0032 BBa_B0032] | 5E [http://parts.igem.org/Part:BBa_R0040 BBa_R0040] | 6I [http://parts.igem.org/Part:BBa_J45199 BBa_J45199] | 1H [http://parts.igem.org/Part:BBa_B0030 BBa_B0030] |
| 1G [http://parts.igem.org/Part:BBa_K314100 BBa_K314100] | 7E [http://parts.igem.org/Part:BBa_K808000 BBa_K808000] | 20O [http://parts.igem.org/Part:BBa_I13453 BBa_I13453] | 19K [http://parts.igem.org/Part:BBa_J45014 BBa_J45014] | 2M [http://parts.igem.org/Part:BBa_B0034 BBa_B0034] |
| 3D [http://parts.igem.org/Part:BBa_K823017 BBa_K823017] | 15N [http://parts.igem.org/Part:BBa_I719005 BBa_I719005] | |||
| 3O [http://parts.igem.org/Part:BBa_K608002 BBa_K608002] | ||||
| 4D [http://parts.igem.org/Part:BBa_K314103 BBa_K314103] | ||||
| 5E [http://parts.igem.org/Part:BBa_K608006 BBa_K608006] | ||||
| 17D [http://parts.igem.org/Part:BBa_K864600 BBa_K864600] |
During the heat shock the heat automatically turned off after one round, wicht we didnt realize at first. So for the bricks from plate 2 and 3, the heat shock temperature is a bit lower (around 37°C). Hence during the plating we used 50µl of transformed competent cells instead of 20µl for the tubes from plates 2 & 3.
We also inoculated methyl salicylate producing cells (received from iGEM) on both Amp & Kan plates.
08/07
Unpatient as we were, we first checked the plates of the transformed competent cells and found out that only 4 petri dishes have small amount of colonies (7E2 top10, 5E3 top10, 1G1top10, 5E3 DH5alpha). Interestingly, cells plated on plates 2 and 3 (the ones where we used 50µl cells instead of 20µl), were most succesful, which means that the lack of colony may be caused by low amount of cells put on the petri dishes. This in turn also proves that the transforming efficiency is quite low. We should repeat this step with 50µl or more cells today.
In the mean time, we also prepared LB agar medium and LB chloramphenicol agar medium to pour the plates.
In the afternoon we inoculated the methyl salicilate strains with medium containing both ampicilline and kanamycin for each AB medium, the volume is 3ml per tube, and we repeated 3 tubes for each AB medium.
Moreover, we transformed the unsuccessful biobricks again into the competent cells.
09/07
We made methyl salicylate producing cell stocks from the inoculation of yesterday and tried to isolate their plasmid.
We also tried to make competent TOP10 and DH5alpha cells using the Inoue method .
10/07
We had a busy lab day today, with 3 main experiments running parallel, so all hands on deck!
In the morning, we first made the gel to separate the plasmid that we extracted yesterday. The gel looked good, the separation is very clear.
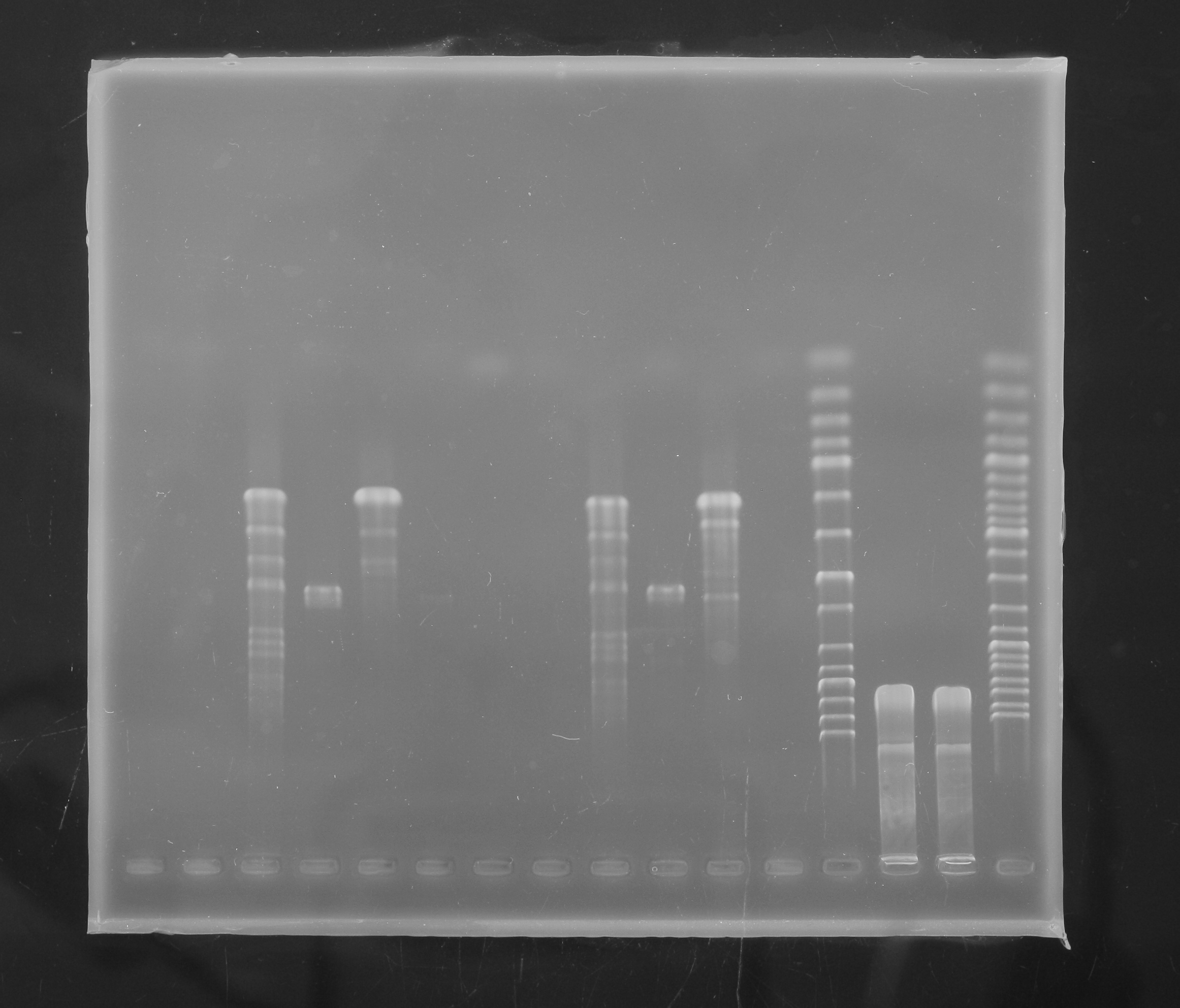
Afterwards, we extracted the plasmid from the gel by using the gel extraction kit and measured the amount of DNA with nanodrop.
We also finished with the Inoue method to make competent cells.
We wanted to do a colony PCR on Streptomyces, so first we prepared the primer stocks for our PCR.
Ingmar extracted the genome from Streptomyces at IMPG using 4 different methods (as Streptomyces is gram negative, it is a bit difficult to extract the genome)
- microwave Streptomyces for 4mins
- Streptomyces in water and 0.2%SDS, 4min microwave
- Streptomyces in water and 1% SDS, 4min microwave
- Streptomyces in TE buffer, 0.2% SDS, 4min microwave
We ran a colony PCR for all these streptomyces + a negative control.
In the afternoon, we also performed the plasmid extraction of all the successfully transformed biobricks.
11/07
Today is a Flemish holiday so the lab was closed unfortunately.
 "
"




