Team:Calgary Entrepreneurial/Project/Technology/
From 2013.igem.org



FREDsense's website works best with Javascript enabled, especially on mobile devices. Please enable Javascript for optimal viewing.
Developing the Technology
Bla bla bla bla bla bla bla bla bla bla bla bla bla
Overview
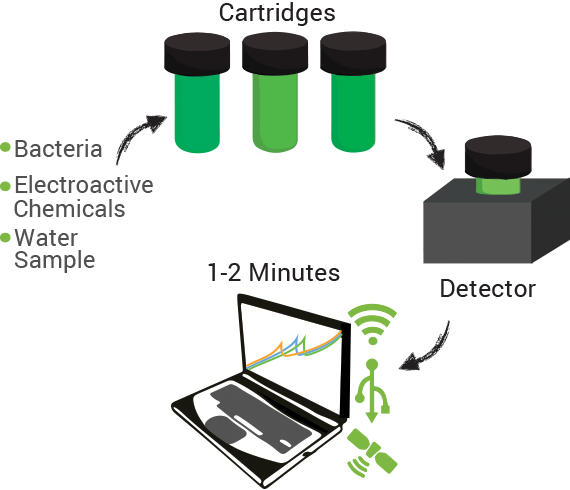
Our product is a toxin sensor for detecting a variety of oil and gas related contaminants in water supplies. The system is portable, on-site, and rapid allowing for monitoring of toxins in the environment much more quickly and easily than present systems allow. It uses a strain of Pseudomonas which has been synthetically designed to react to numerous toxins in the environment. These toxins result in the production of enzymes capable of producing electro-active compounds. Our system detects these electroactive chemicals and measures the amount produced using a simple computer, USB, WiFi, or GPS based interface.
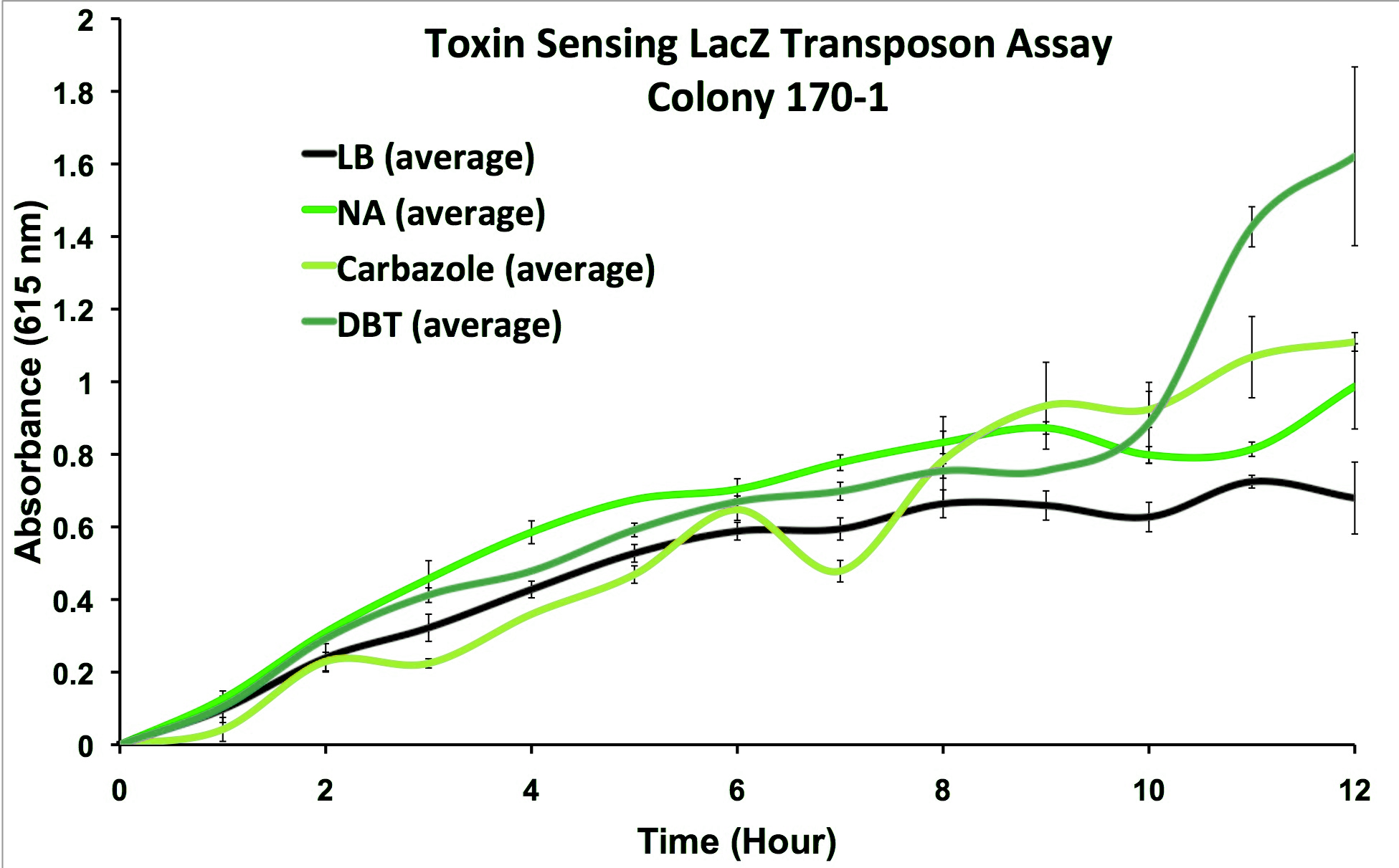
Presently we have shown our strain to be able to detect three relevant oil sands toxins (naphthenic acids, carbazole, and dibenzothiophene) and that it does not react to other stresses such as hydrogen peroxide or healthy compounds (decanoic acid). We have demonstrated that we can measure the production of our electrochemical analytes and working to demonstrate that we can use multiple enzyme/electroactive compounds simultaneously to make our system more effective. Therefore, we have a proof-of-concept technology which will require further refinement prior to developing a prototype system.
Technical Description of the Technology
Sensing Microorganism:
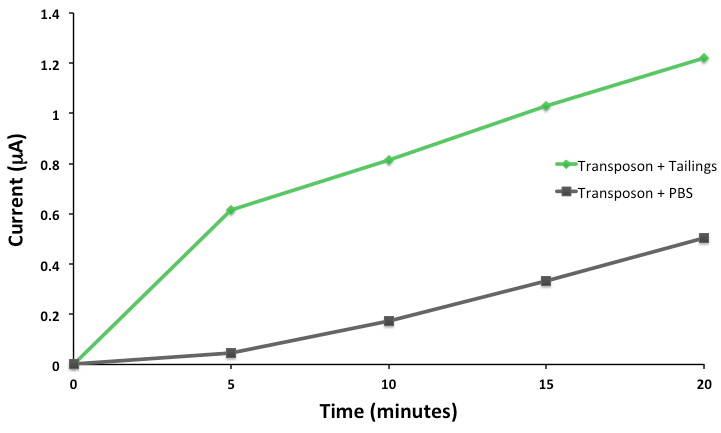
A transposon screen using an electrochemical reporting enzyme (lacZ) was performed on Pseudomonas fluorescens Pf-5 (a native tailings pond organism shown to degrade oil sands toxins), and strains were found which respond to general oil sands toxins (sulfur-heterocycles, nitrogen heterocycles, and naphthenic acids were specifically shown to elicit a response higher than baseline, without responding to general hydrocarbon compounds and stress agents). This response consisted of production of a reporter enzyme capable of cleaving a substrate which could then give an electrochemical response. Further testing on one strain using actual tailings pond samples showed a higher than baseline response, and the organism was capable of survival in the toxic substrate. The sensing elements themselves have not been identified or characterized as of yet, and the system has yet to be multiplexed, providing only one output for general toxin response.
Electrochemical Enzymatic Reporting System:
The oxidation potential chlorophenol red (CPR), para-diphenol (PDP), and para-nitrophenol (PNP) as substrates was tested. These compounds are conjugated with their sugars to form CPR-β-D-galactopyranoside (CPRG), PDP-β-D-glucopyranoside (PDPG), and PNP-β-D-glucuronide (PNPG). It was found that when measured using cyclic voltammetry, the oxidation potentials of the three substrates were distinct and could be visualised separately on the resulting graph, showing the potential for multiplexing the system.
Three hydrolase enzymes were identified which have been shown to act on these substrates; beta-D-glucoside glucohydrolase (bglX gene), beta-glucuronidase (uidA gene), and beta-galactosidase (lacZ gene). Commercial versions of these enzymes were tested and it was shown that by cleaving their substrates an electrochemical signal could be detected by a potentiostat. These enzymes were put into standardized plasmid backbones for expression in Escherichia coli under control of an inducible promoter for testing.

Hardware, Software, and Prototype Design:
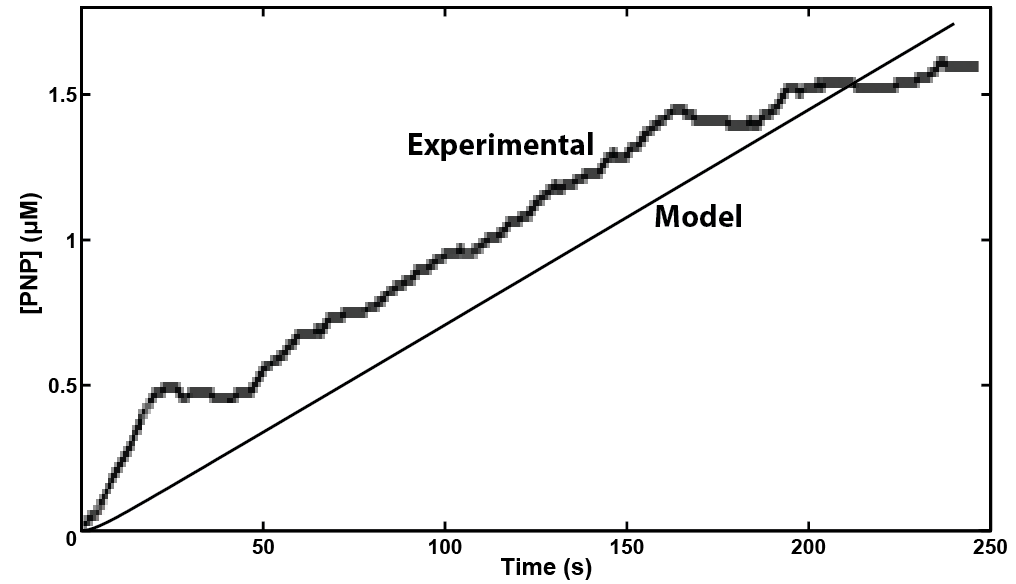
A kinetic model was built for UidA in MatLab, predicting that a response could be detected within 4 minutes of adding the substrate to the enzyme when read electrochemically. This experiment was then performed in the wetlab by inducing UidA expression, and results were consistent with the models prediction.
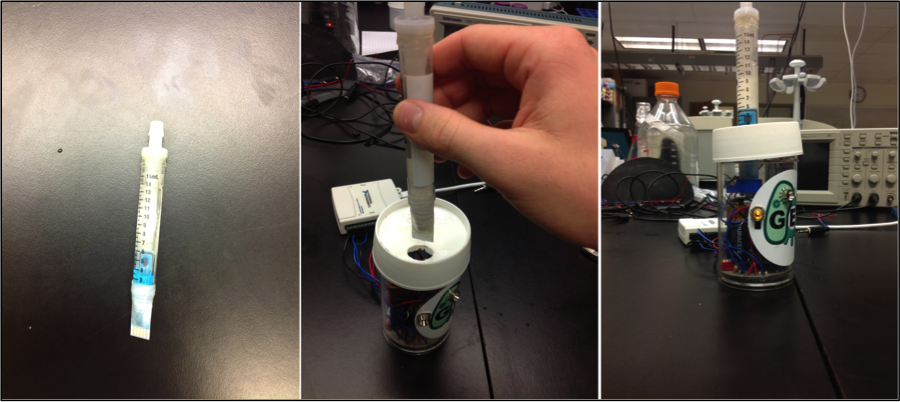
As well, an inexpensive, small, and portable potentiostat was designed and developed, with required circuitry being printed onto a small circuit board. While larger versions of the potentiostat were shown to function well, an error in the design of the circuit board renders the latest prototype nonfunctional at the moment, preventing further testing and refinement from being done. A custom software package within the MatLab platform was also developed in order to interpret and display the data obtained from electrochemical measurement.
Finally, a prototype device was designed, consisting of a small can-sized container where circuitry would be housed, a slot where a cartridge could be inserted into the housing, and a cartridge where the electrodes, analytes, and microorganisms could be housed with a lid allowing sample injection. The device contained LED lights in order to indicate what step of the reading was currently running as well as to indicate when analysis was finished.
Technical Implementation Plan
These points are listed in order of priority with the earliest described experiments being most critical in evaluating the commercial potential of our technology.
Demonstration of CV response of 2 to 3 simultaneous hydrolase products from E.coli chassis (all hydrolase enzymes to be expressed endogenously). While this has been demonstrated in vitro, in vivo characterization is still required. This is crucial in evaluating if detection of multiple signals simultaneously is possible.
Comparison of hydrolase induction and activity in E.coli and P. fluorescens with inducible lacZ, uidA, and bglX. This involves characterization of individual hydrolase enzymes when expressed on plasmids. The hydrolases used in our system must be specific (not cross react with other sugar moieties) and capable of recognizing the substrates used in our electrochemical system. Should any hydrolase enzymes fail to function as expected, others will need to be cloned and characterized.
Synthetically engineer multiplexed systems in E. coli and Pseudomonas chassis. Combine inducible hydrolase enzymes in E. coli and/or P. fluorescens. This will demonstrate that all three signals can still be detected in vivo with inducible expression systems, as would be found in our final system.
Validation of the transposon strain we have created. Further characterize the clones derived from a screen of tailings pond bacteria, particularly for toxin sensitivity as well as specificity to the class of chemicals that they respond to.
Fabrication of physical prototype. The laboratory characterization of this system will need to be translated to a field-ready portable device. Design of the portable device will require the help of engineers and fabrication facilities to make testable prototypes. The bacteria chassis will need to be packaged in such a way to enable utilization in the field. Integration software needs to be designed to translate the readings into toxin concentrations. Lastly, containment of the genetically modified organism must be considered to prevent contamination the environment.
Optimization of chemical composition and the protocol. Evaluate buffer conditions that will result in the most consistently accurate signal for evaluating toxicity with a variety of samples and complex mixtures. Establish the detection threshold of the system and characterize system performance in a variety of conditions.
Optimization of the field protocol using the prototypes. The protocol will require comparison of on-site sampling to thresholds of detection that have been characterized for our bacteria. We will need to analyze the breadth of detection and the accuracy of the system in a variety of sample solutions and compare our detection methods to competitors systems.
Further transposon screening. With the intent of eventually expanding our system’s detection capabilities and extending into new markets, we will generate detectors for relevant compounds. This will expand the repertoire of detectable compounds from the general toxin sensor we have now and be the next generation products needed to further develop our company.
Responding to Technological Change
The current technology has been carefully designed in order to easily facilitate the production of next generation products. FREDsense’s unique sensing platform is easily adaptable to monitor a plethora of different compounds of interest using different engineered bacterial strains. Second generation products are being developed including detector products specific to unique classes of chemicals including naphthenic acids and hydrocarbons. As these are specific compounds of interest for many oil sands-related processes, detectors capable of monitoring them with specificity are sought-after. These detectors will make use of FREDsense’s novel generation one sensing platform coupled with newly developed bacterial strains. Being able to directly build upon the existing platform will help ensure FREDsense’s new products can be designed quickly and efficiently so as to have a quicker entry to market than generation one products.
With the introduction of more specific and diverse sensors, this will lead the way for multiple-output detector systems. These will include detectors that can monitor multiple different classes of compounds such as combinations of naphthenic acids, hydrocarbons and general toxins simultaneously. As many locations would require monitoring of a variety of compounds of interest at one sampling time, these second generation sensors will allow one water sample to be taken in order to monitor that sample for several toxins. This will allow for quicker sample extraction and faster results. Again, these products will make use of the generation one electrochemical sensing platform with new engineered bacterial strains.
As with generation one, all later generation products will include integrated controls as well as the speed and reliability of the generation one sensing platform. The modular design of FREDsense’s technology will allow the company to adapt quickly and easily to changes in the regulatory landscape in the oil sands market as well as expand into different markets. This will allow FREDsense to expand the breadth of its detection products without investing significant resources into research and development.
Regulatory Hurdles
Biotechnology products in Canada are regulated by three federal agencies: Health Canada for foods, Canadian Food Inspection Agency (CFIA) for seeds and livestock feed, and Environment Canada for new substances intended for environmental release. Our product thus falls under regulation set out by the later. This regulation falls under the Canadian Environmental Protection Act (jointly administered by Environment Canada and Health Canada). Environmental Technology Verification is also required for our technology to be used in this industry. Risk is assessed based on relative toxicity as well as relative amount of exposure to the environment. Specific products are assessed on a one-to-one basis through a dialogue with Environment Canada and Health Canada.
 "
"