|
Overview
Winter
Spring
Summer
Fall
|
1. Goals for the week
- Our goals for the past few weeks were to establish that the basic procedures for our direct evolution method works. Specifically, we can grow T7 in liquid culture with E. coli, amplify phage to create a high titer, select for smaller phage using concentrated top agar, and propagate phage from a single plaque.
2.Experiments and results
Phage growth in liquid culture (5.3 Phage Amplification/Purification)
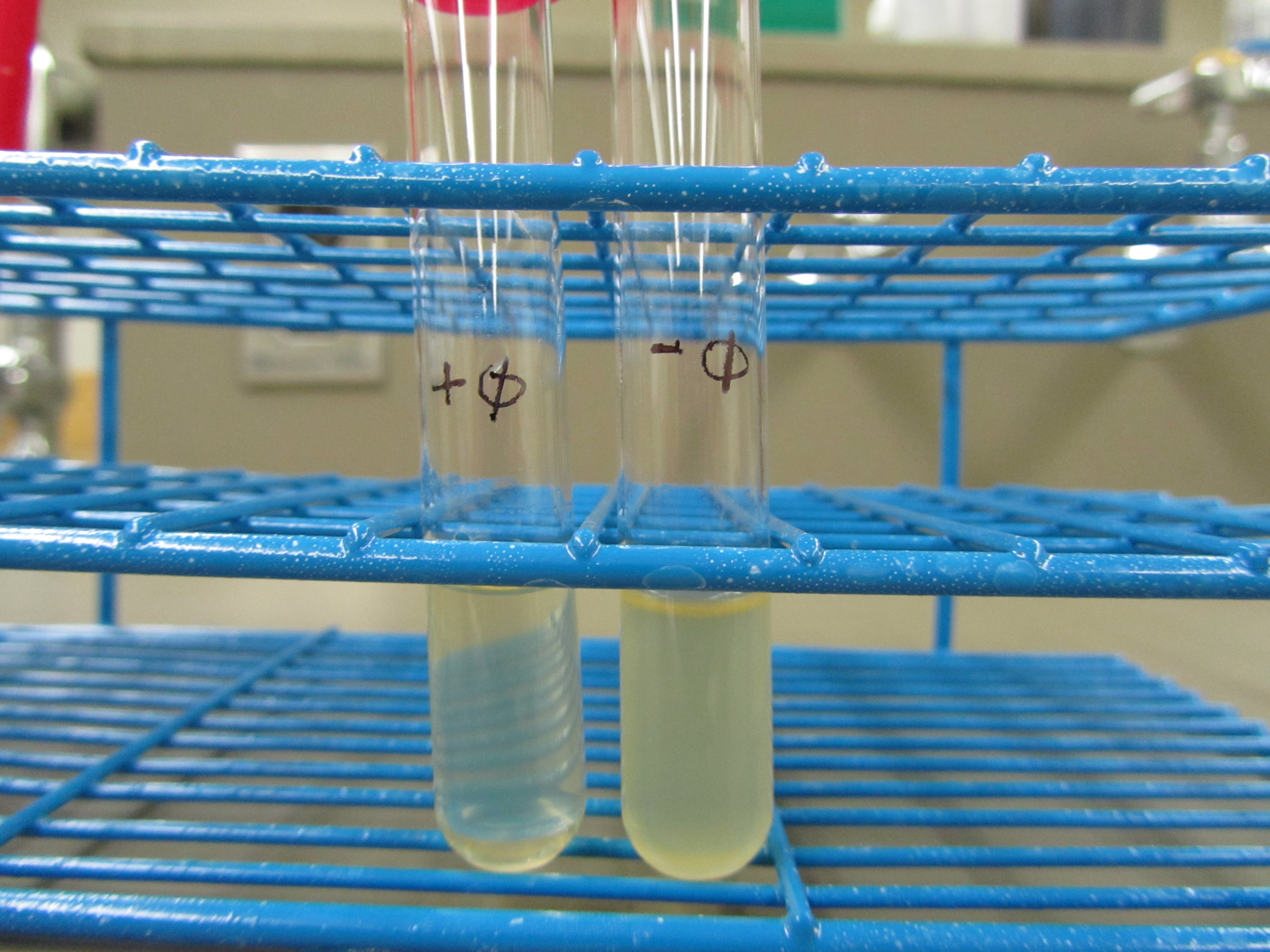
- Phage liquid culture set up
- + phage: 4mL LB + 1mL E. coli overnight + 100μL phage received stock
- - phage: 4mL LB + 1mL E. coli overnight
-
- Obvious clearage was seen for the + phage test tube
- Spot test result
- We purified the phage via centrifugation and adding chloroform. This batch of phage is stored as stock 5.3 in the fridge.
-
- This confirmed our conclusion that we are able to grow T7 in liquid culture with E. coli BL21.
Amplifying phage (5.6 T7+ Liquid Culture Phage Concentration Test)
- We tested whether adjusting the amount of phage that we add to liquid culture will produce different spot test results. Purified phage were labeled stock 5.6 in the fridge.
- stock 5.6 10: 4mL LB + 1mL E. coli overnight + 10μL phage from 5.3 stock
- stock 5.6 100: 4mL LB + 1mL E. coli overnight + 100μL phage 5.3 stock
- stock 5.6 1000: 4mL LB + 1mL E. coli overnight + 1mL phage 5.3 stock
-
- Stock 5.6 10 has the highest titer. To amplify phage to a high titer, we should consider lowering the number of phage particles we add to liquid culture.
Selection test
- We tested our proposed method of selecting for small phage using concentrated top agar (4.26 T7 Phage Selection Method).
- 0.5mL of E. coli + 20 μL -5 received stock + 20min incubation + 5mL of agar at various concentration
-
- As the concentration of top agar increases, the number of plaques and the size of the plaques both dropped. This suggested that our method of selection should work.
- We tested ×8 top agar and even fewer plaques formed on it (5.3 T7 Phage Selection Method Test):
-
- However, concentrated top agar solidify so fast that we weren’t able to get it even spread on the plate. We decided to try warming up the plates in 37°C incubator for 3 hours before use (5.9 T7 Phage Selection Method Test #2). Our plates are much prettier this time.
:
Stabbing test (5.5 Amplification from a plaque test)
- We wanted to see whether or not we can grow liquid culture from a single plaque.
- 4mL LB + 1mL E. coli overnight + 1 stab at a plaque from a ×6 plate in the 5.3 T7 Phage Selection Method Test. The plaque in question is circled in the photo above. After incubation for approximately 24 hours, phage solution was purified and spot test was performed. The remaining phage solution is labeled stock 5.4 in the fridge.
-
Others
- We have designed primer for amplifying and sequencing T7 capsid gene.
- We have also designed detailed procedure for applying mutagen and PCR.
3. Next Steps
- We will be starting our mutagenesis and PCR experiment next week.
- We are also proposing to take EM with the wild type phage stock we have.
|
 "
"