Team:BYU Provo/Notebook/LargePhage/Summerexp/Period4/Dailylog
From 2013.igem.org
(→8/21/13) |
Brymerr921 (Talk | contribs) |
||
| Line 40: | Line 40: | ||
===8/28/13=== | ===8/28/13=== | ||
| + | Today we got our mutagenesis back from the purification group. It came in 21 fractions. Fractions 1-9 were from below the band, 10-12 were where the band was, 13-19 were above the band, and 20-21 were where the bacterial debris was. I did a spot test of fractions 1-6 and 20-21 at 10^0 and 10^-2 (5 uL of each on a plate). | ||
===8/30/13=== | ===8/30/13=== | ||
| Line 47: | Line 48: | ||
===9/4/13=== | ===9/4/13=== | ||
| + | Results from 8/28/13: Fractions 20 and 21 produced clearage on 10^0, and many small plaques at 10^-2. #4 had one plaque on 10^-2, and #1 had one plaque on 10^0. | ||
| + | Today we infected 0.5 mL of E. coli with: 100 uL of #1 10^0, 100 uL of #4 10^0, 10 uL of #20 10^-2, 10 uL of #21 10^-2, and for controls we did 10 uL of T4 wild type 10^-6 and 1 uL of T4 10^-7. We also plated a lawn of E. coli as a control. | ||
===9/6/13=== | ===9/6/13=== | ||
Revision as of 22:29, 4 September 2013
Contents |
August
8/16/13
8/19/13
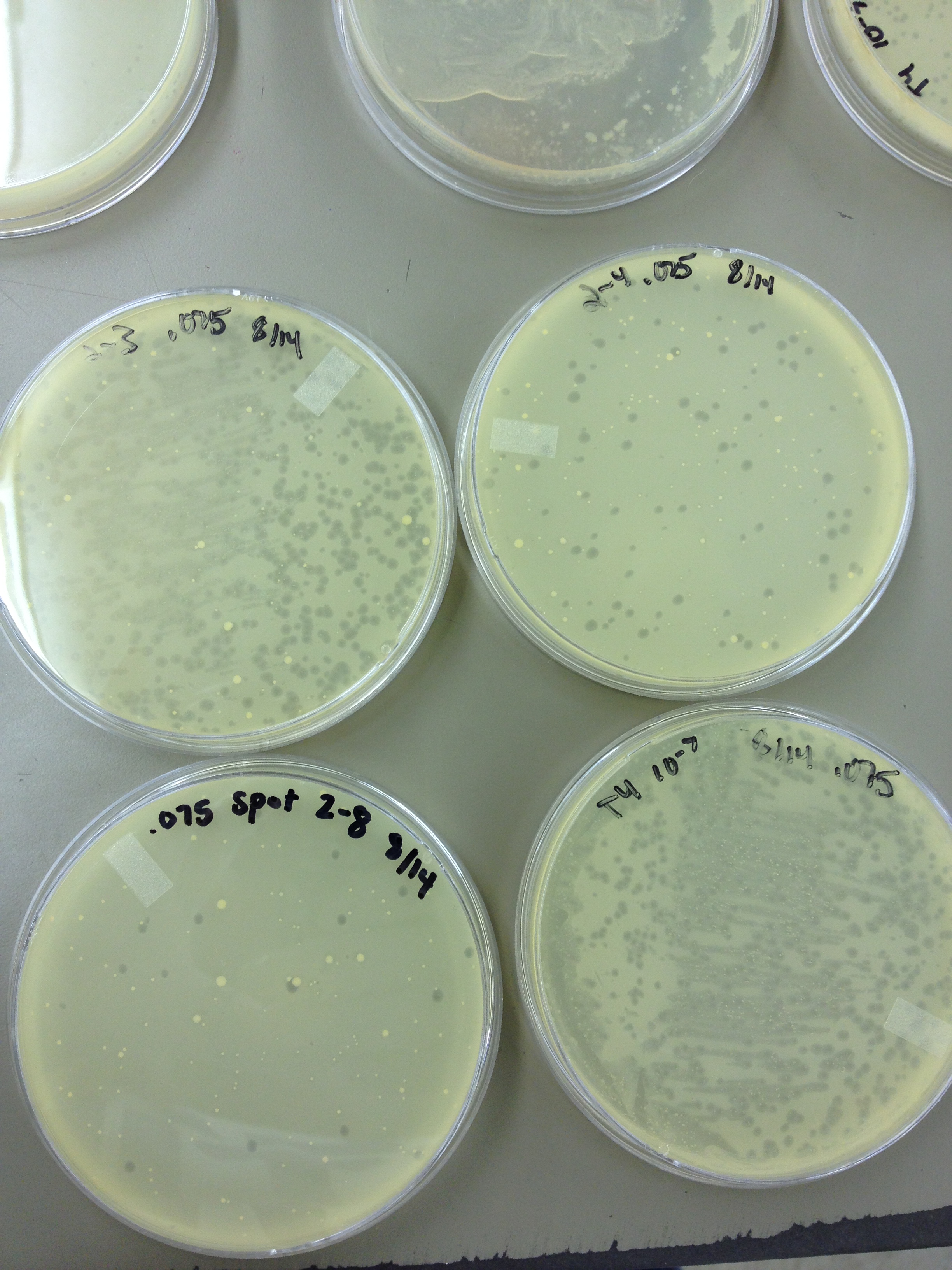
Today we started to do titers on the .5 mL fractions that were taken from the Cesium Chloride gradient. In order to get a general idea, and save some time, we did every other fraction.
We also did a spot test to verify that there was/ was not phage in the lower and higher dilutions as we suspected.
8/20/13

 Today started the dilution series for the other half of the fractions from the cesium chloride gradient, so it will be ready when we want to plate them out.
Today started the dilution series for the other half of the fractions from the cesium chloride gradient, so it will be ready when we want to plate them out.
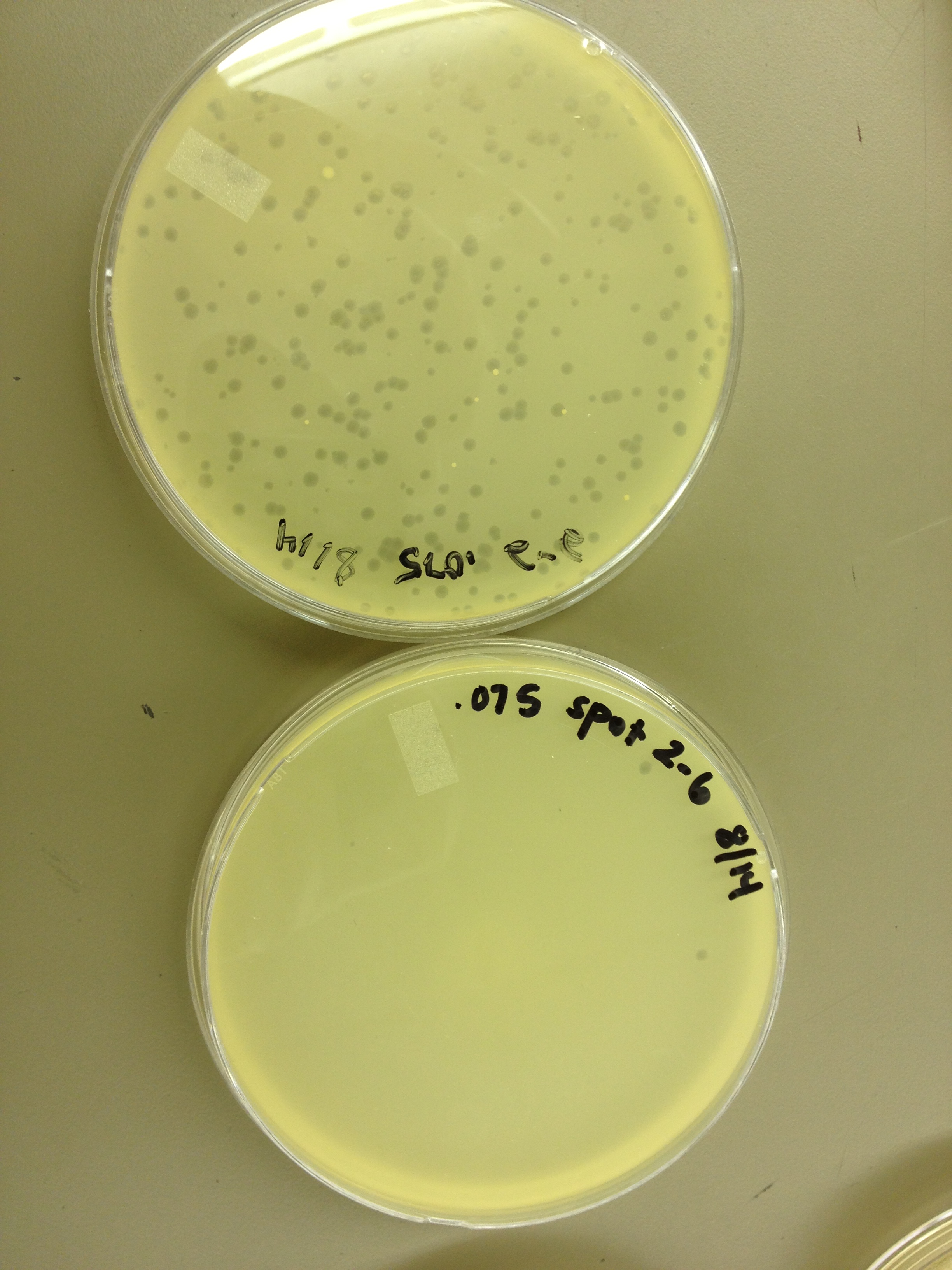
From the spot test and plating of the dilution series, the fractions look like this: Plate 1: 10^-4 Plate 3: 10^-2 Plate 5: 10^-2 Plate 7: 10^-2 Plate 9: 10^-2 Plate 11: 10^-2 Plate 13: 10^-4 Plate 15: 10^-4 Plate 17: 10^-4
8/21/13
Today we started a new mutagenesis experiment. The Protocol being:
In a 250 mL flask, add 25 mL LB broth, 20 ug/mL adenine, and 1 loop of E. coli bacteria. Let incubate on shaker at 37C for ~2 hours until moderately turbid. At this point, you can pellet the bacteria and resuspend it in minimal (M9) media or leave it in the LB broth (unpelleted). At this point, add another 20 ug/mL of adenine, 20 ug/mL of uracil, 200 ug/mL of bromodeoxyuridine (mutagen), and the appropriate amount of phage (we use 1x10^9 pfu) for the bacterial concentration you have. Let incubate on shaker at 37C for 3 hours.
After 3 hours, transfer 5 mL of the culture from the flask to a capped test tube. Add 1 mL of chloroform, shake gently, and let it sit for 5 min. If it clears, the culture is ready to harvest. Pellet the bacteria/phage culture from the flask at 3500 rpm, and resuspend the pellet it in 2 mL of LB broth. Large phage group: discard the supernatant. Small phage group: keep the supernatant and compare the titer of the supernatant with the lysed pellet (follow the following instructions. Transfer resuspended pellet to a capped test tube, add 3 mL of chloroform, 10 uL of 1 M MgCl2, and 40 uL of nuclease. Shake gently and incubate at room temp for 30 mins. Pellet at 2700 rpm for 10 minutes, then transfer the supernatant to a new test tube. This is your high titer lysate.
Today we are also doing a spot test as an initial test for the even numbered fractions from the gradient.
8/23/13
8/26/13
8/28/13
Today we got our mutagenesis back from the purification group. It came in 21 fractions. Fractions 1-9 were from below the band, 10-12 were where the band was, 13-19 were above the band, and 20-21 were where the bacterial debris was. I did a spot test of fractions 1-6 and 20-21 at 10^0 and 10^-2 (5 uL of each on a plate).
8/30/13
September
9/2/13
9/4/13
Results from 8/28/13: Fractions 20 and 21 produced clearage on 10^0, and many small plaques at 10^-2. #4 had one plaque on 10^-2, and #1 had one plaque on 10^0.
Today we infected 0.5 mL of E. coli with: 100 uL of #1 10^0, 100 uL of #4 10^0, 10 uL of #20 10^-2, 10 uL of #21 10^-2, and for controls we did 10 uL of T4 wild type 10^-6 and 1 uL of T4 10^-7. We also plated a lawn of E. coli as a control.
9/6/13
9/9/13
9/11/13
9/13/13
9/16/13
9/18/13
9/20/13
9/23/13
9/25/13
9/27/13
9/30/13
 "
"