Team:Dundee/Project/Detector
From 2013.igem.org
The Detector
Aim: To engineer the B. subtilis receptor PrkC to respond to microcystin
B. subtilis forms resistant structures called spores in order to survive harsh environmental conditions. In order for the spores to recognise that the conditions have again become favourable for growth the spores have to monitor the extracellular environment. This is done via a number of inner-membrane receptors described as germinant receptors. PrkC is an example of a germinant receptor and it binds to cell wall associated peptides.
Sensing cell wall peptides & conditions that are permissive for growth
Actively growing cells turnover cell wall components and these can thus be found in the extracellular milieu. So by sensing cell wall components, through the PrkC receptor, the spore can tell that other cells are growing in the nearby environment. This is how the PrkC receptor can signal to the spore that conditions are permissive for growth.
PrkC receptor activation
PrkC receptor activation triggers a process called germination which is the conversion of the spore back into an actively growing cell.
PrkC receptor
The PrkC receptor has 4 extracellular domains PASTA 1, 2 and 3 which are capped by a C-terminal domain and this sits on the outside of the spore inner membrane. The 3 PASTA domains are implicated in binding of the cell wall components and are thus described as the ligand binding domains (fig 1).
PrkC receptor to detect microcystin
We hope to detect microcystin by replacing the 3 ligand binding domains with the human protein- protein phosphatase 1 (PP1) (fig 2).
We hope that when the microcystin binds to PP1 this will still result in activation of the downstream pathways controlled by the native PrkC receptor. Additionally, we hope to have our B. subtilis strain constitutively expressing green fluorescent protein so that when it is relieved from dormancy it will fluoresce and this will hopefully be detectable with our electronic Moptopus device.
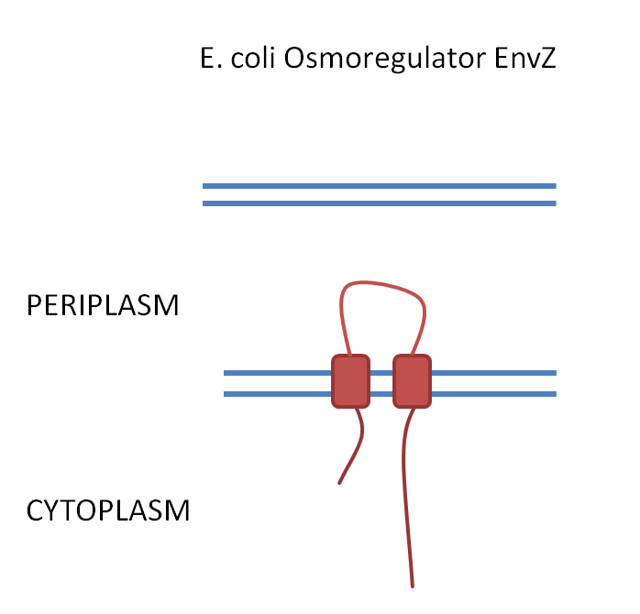
Figure 1. .
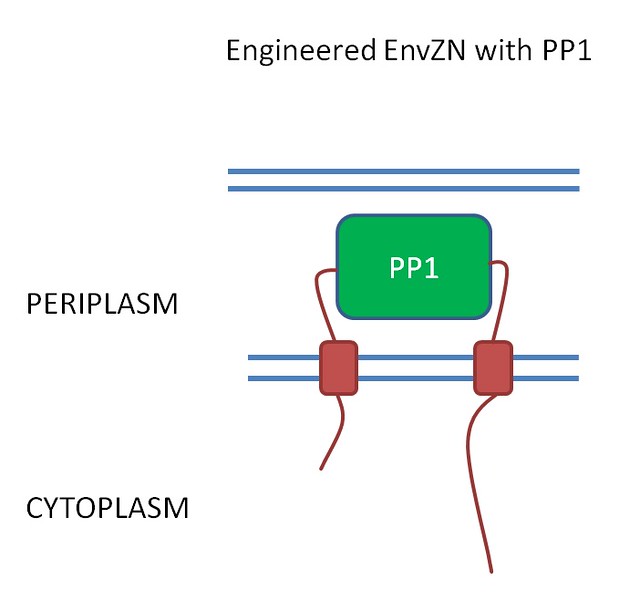
Figure 2. .
Progress so far...
We are currently in the process of cloning this receptor and we are having some difficulty. We have successfully cloned the N-terminal part of the receptor and are currently in the process of adding on the PP1s which we will be doing by suicide ligation. The final step after adding the PP1s will be to add on the C-terminal domain.
Aim: To engineer the E. coli EnvZ sensor kinase to respond to microcystin
The EnvZ system is a signal transduction system composed of two parts and is, therefore, described as a two-component regulatory system. Part 1 is the sensor kinase protein located in the membrane of the cell and Part 2 is the response regulator protein. The native EnvZ sensor detects changes in osmolarity.
EnvZ sensor kinase
The sensor kinase EnvZ detects a signal from the environment and auto-phosphorylates. The phosphoryl group is then transferred to the response regulator OmpR. OmpR is a DNA-binding protein.
E.coli is a gram-negative bacteria which means that is has both an inner and outer membrane. The EnvZ sensor sits on the inner membrane (Fig 3).
EnvZ sensor to detect microcystin
What we want to do for our project is replace the periplasmic domain of EnvZ with the PP1 protein (Fig 4). We hope that when microcystin binds to PP1 then it will activate the receptor.
This will lead to the phosphorylation and activation of the DNA binding protein OmpR. We will also express in our engineered bacteria a DNA construct encoding GFP that’s expression is under control of the OmpR protein.
So our cells will turn green in the presence of microcystin and in this way act as a microcystin detector.
Figure 3. .
Figure 4. .
Progress so far...
WSo far we have successfully cloned the N-terminus with PP1 and we are in the process of adding on the C-terminus. We have also found an OmpR regulated construct in the distribution kit and we have transformed cells to make more of this part. We have also identified GFP in the kit and we will try and join these 2 parts together to make our reporter construct.
Characterisation of our receptors
We will be looking to quantify how many of our PrkC receptors are expressed on the surface of the spores and also how many EnvZ sensors are present on our E. coli cells.
We will be able to get hold of some microcystin and we can use this to bind and activate our receptors. We will then measure the amount of fluorescence by flow cytometry or microscopy. We can then quantify the expression of GFP in relation to how much microcystin is presented to our cells. By using values for how many receptors we have on each cell we can calculate the efficiency of our detectors and hopefully use all this information in order to quantify the effectiveness of our detector.
 "
"