Header
Traditionally when people get sick they swallow a pill or get an intravenous injection. The drug gets distributed through the whole body of the patient, and can cause severe side effects. The idea of targeted drug delivery is to increase the concentration of the drug at the specific sick tissue compared to the drug concentrations in the healthy tissue. This could be used to treat various diseases, but especially to treat tumor conditions. In theory the side effects of the drugs would be less severe also there wouldn’t be the spikes of drugs in the blood system, and finally the amount of drugs could be reduced. One big drawback of targeted drug delivery is that it doesn’t exist for many drugs, it needs to be developed for each drug separately and that is obviously quite expensive.
Our team aims to create a highly adaptable smart drug delivery system, which could be used for several applications and alternatives in disease treatment. The design makes it so flexible that it could even be possible to adapt it to the single patient’s needs. By being highly adaptive, the system we propose would also reduce the cost of targeted drug delivery.
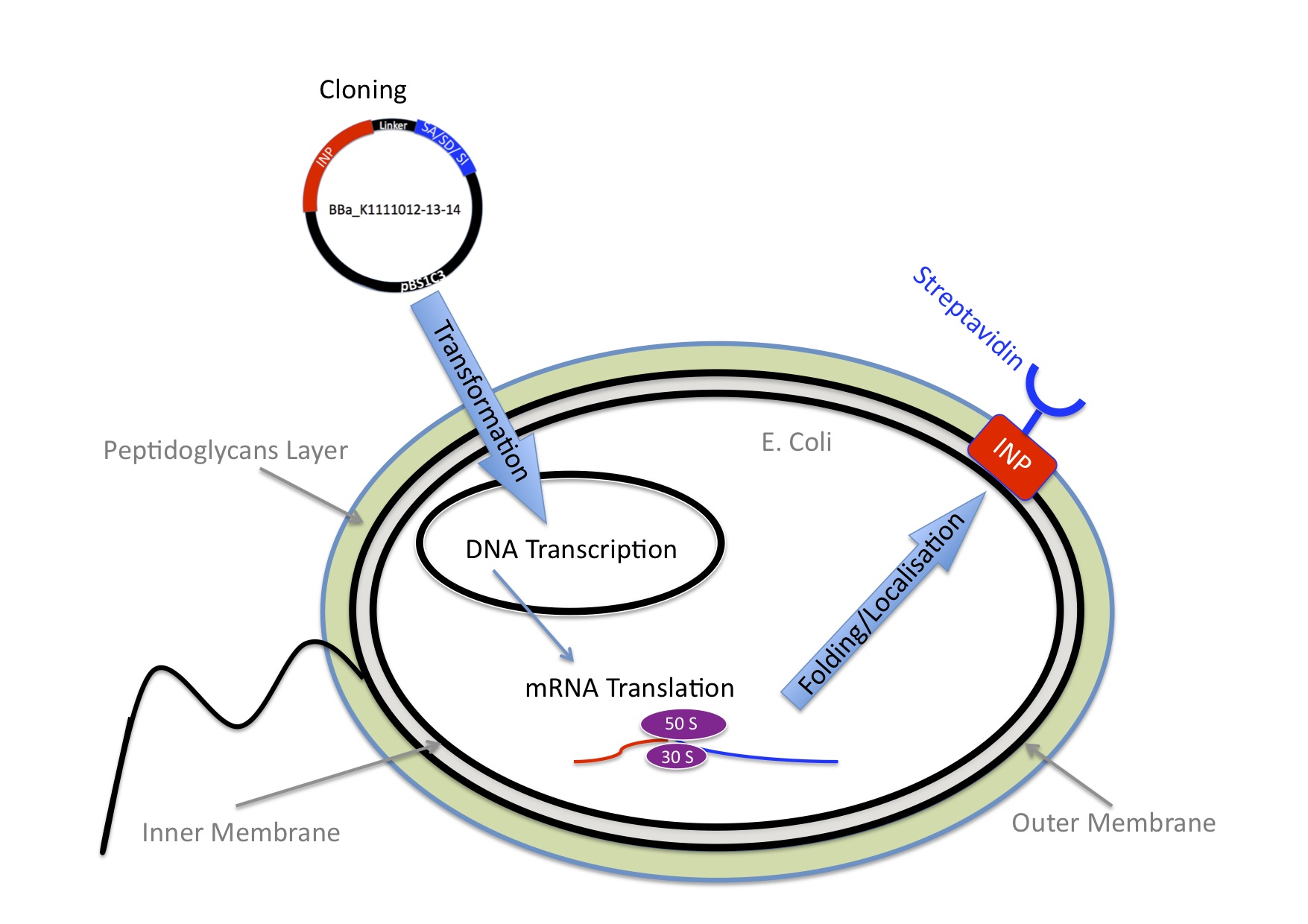
Given the limited time we only wanted to make a proof of principle. We used E.coli as model organism, using the principles of synthetic biology we aimed to engineer E.coli that expresses streptavidin on their surface as well as being able to sense either changes in pH or arabinose. Those bacteria would be able to bind biotinylated nanoparticles.
The nanoparticles are made out of gelatine, this kind of nanoparticles has been shown to be able to transport xxy. They were biotinylated using activated biotin. We invite you to read more about nanoparticles. To assemble the nanoparticles with the bacteria, they only need to be in the same tube, because the interaction between the biotin and streptavidin is very strong.
To make the bacteria express streptavidin on their surface, we engineered a plasmid that encodes a fusion protein between streptavidin and ice nucleation protein from pseudomonas syringae, which serves as an anchor for the streptavidin to the outer membrane. Read more about this part of the project. At this point the bacteria would be able to transport a nanoparticle, but on its own this wouldn’t make any sense.
The crucial point of our project is that E.coli was genetically engineered to sense changes in pH or arabinose. Read more about sensing. Sensing this changes triggers the production of enzymes that degrade the nanoparticles, gelatinase and MMP2 (Luisa, please add the correct name). So the drug will be released exactly where it is needed to treat the patient.
As mentioned before we only aimed to make a proof of principle, eventually it would be necessary to develop the system in another organism, to add safety mechanisms, and develop different sensing plasmid. Read more the future applications we imagine.
 "
"