|
|
| (74 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | {{Template:EPFL2013Header}} | | {{Template:EPFL2013Header}} |
| | | | |
| | + | This section provides a short summary of our main experimental achievements. |
| | | | |
| - | The nanoparticles were decided to be composed of gelatin, and a two step desolvation protocol was successfully used to obtain them. We were able to prove their presence by DLS method, giving an average size of nanoparticles of 200nm.
| |
| - |
| |
| | <br> | | <br> |
| - | [[Image:Team-EPF-Lausanne_results_NPs_DLS1.jpg,|thumb|400x|center|Figure 1 : DLS experiment results]]
| + | <br> |
| | | | |
| | + | ==Nanoparticles:== |
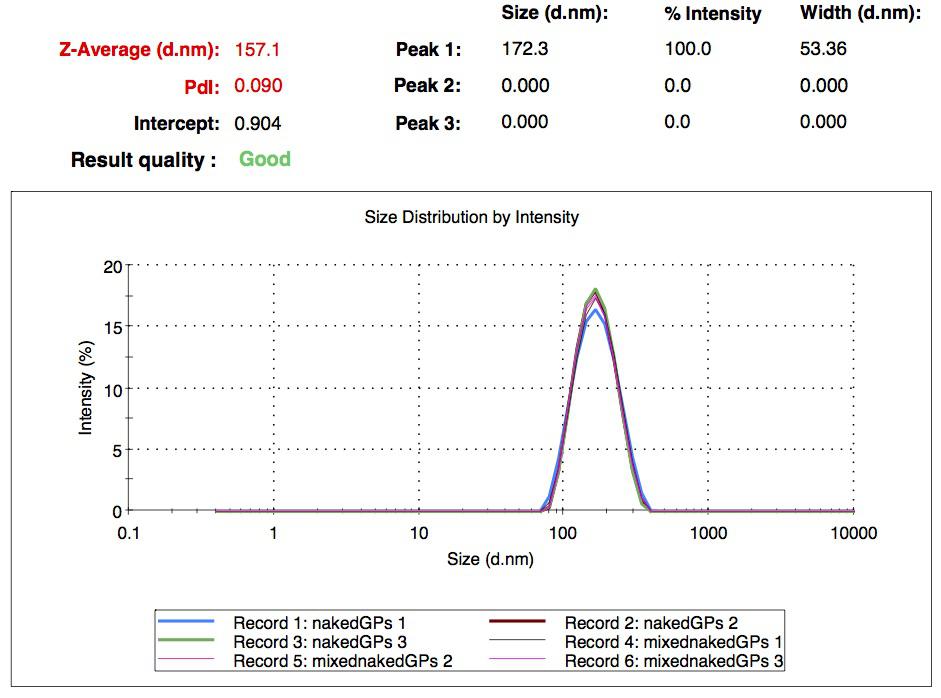
| | + | We successfully synthesized different batches of nanoparticles, whose mean diameter is in a range between 200 nm and 300 nm. We ended up with seven different collections of samples: simple naked gelatin nanoparticles, simple biotinylated nanoparticles, CY5-labeled biotinylated nanoparticles, rGFP loaded nanoparticles (naked and biotinylated) and FITC-Dextran loaded nanoparticles (naked and biotinylated). All of them were characterized by DLS. |
| | + | These experiments show that two cargo transport strategies are possible: an external labeling (the one we used with CY5) or an internal loading (with FITC-Dextran and rGFP). |
| | | | |
| - | <br>
| + | [[Image:Team-EPF-Lausanne_results_NPs_DLS1.jpg|thumb|250px|left|Figure 1: DLS experiment results of the first nanoparticles we obtained: their mean diameter is a bit below 200 nm.]] |
| | | | |
| - | The biotinylation was chemically made with activated biotin and proved with an Elisa-like method.
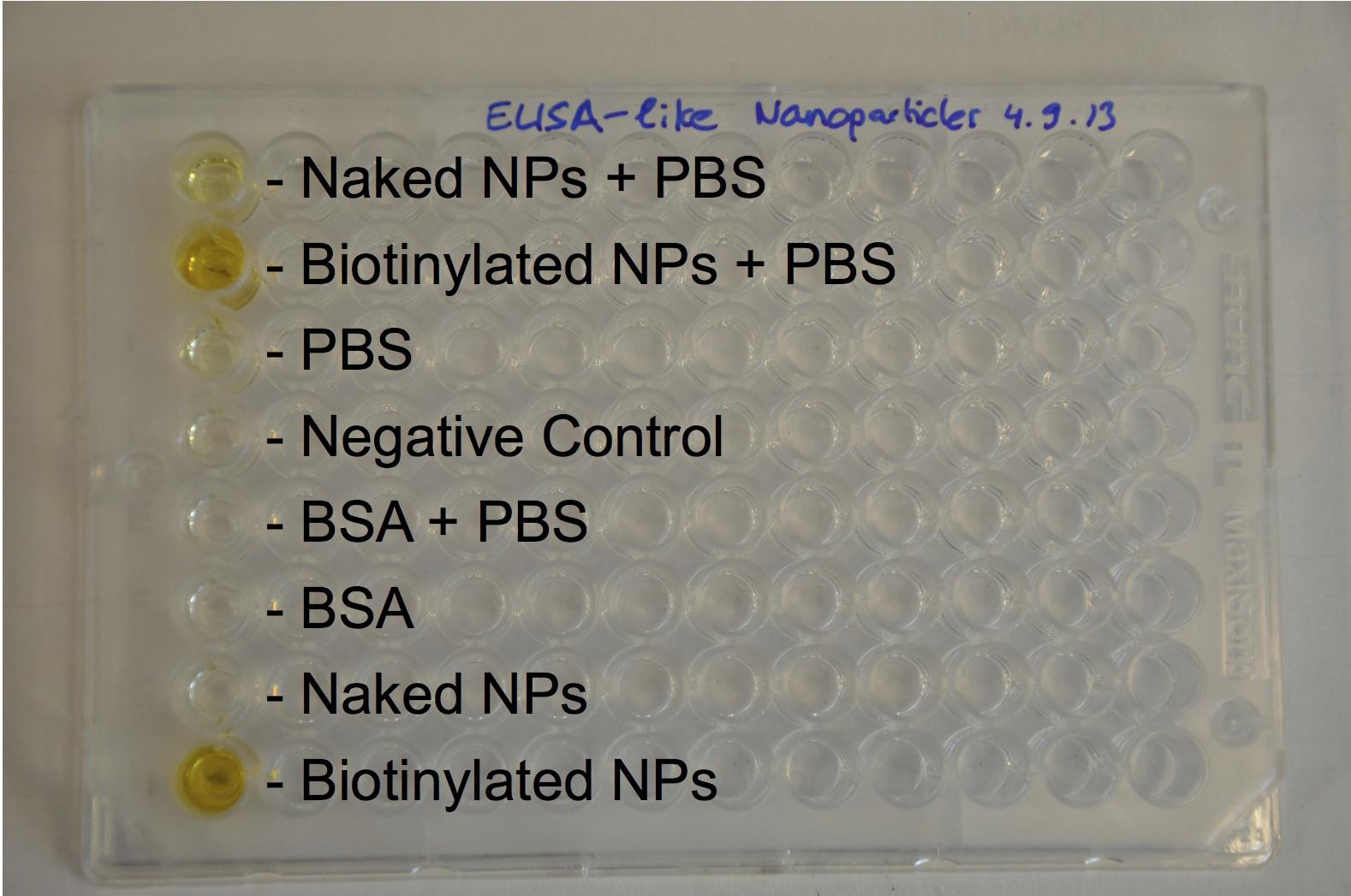
| + | [[Image:Team-EPF-Lausanne_results_ELISA-like2.jpg|thumb|250px|left|Figure 2: ELISA-like assay. The high absorbance in wells B and H (containing biotinylated nanoparticles) was quantitatively detected using a plate reader. It showed that the nanoparticles were well biotinylated.]] |
| - | <br>
| + | |
| - | [[Image:Team-EPF-Lausanne_results_ELISA-like2.jpg| thumb|400x|center|Figure 1.]] | + | |
| - | <br>
| + | |
| - | Biotinylated nanoparticles were dyed and observed under microscope.
| + | |
| - | <br>
| + | |
| - | [[Image:Team-EPF-Lausanne_results_confocal_positive.jpg|left|300px]]
| + | |
| - | <br>
| + | |
| - | Once proved that we could obtain nanoparticles, the loading was tried with two distinct fluorescent molecules : GFP, relatively small, and FITC-dextran, with a large MW due to sugars. FITC fluorescent images showed the incorporation of the molecule, while nanoparticles were not able to incorporate GFP, maybe due to its small MW or the acetone steps during the protocol.
| + | |
| - | Biotinylation was proved to be still possible after loading with FITC, what we did with an Elisa-like assay.
| + | |
| - | <br>
| + | |
| | | | |
| - | For sensing part, two pH-dependent and a constitutive promoter were inserted in front of a superfolded GFP sequence (Biobrick BBa_I746916 ). The inserts, consisting in the three different promoters, as well as the insert were isolated and amplified successfully by PCR. A gel was made to assess it and the sizes were the expected ones.
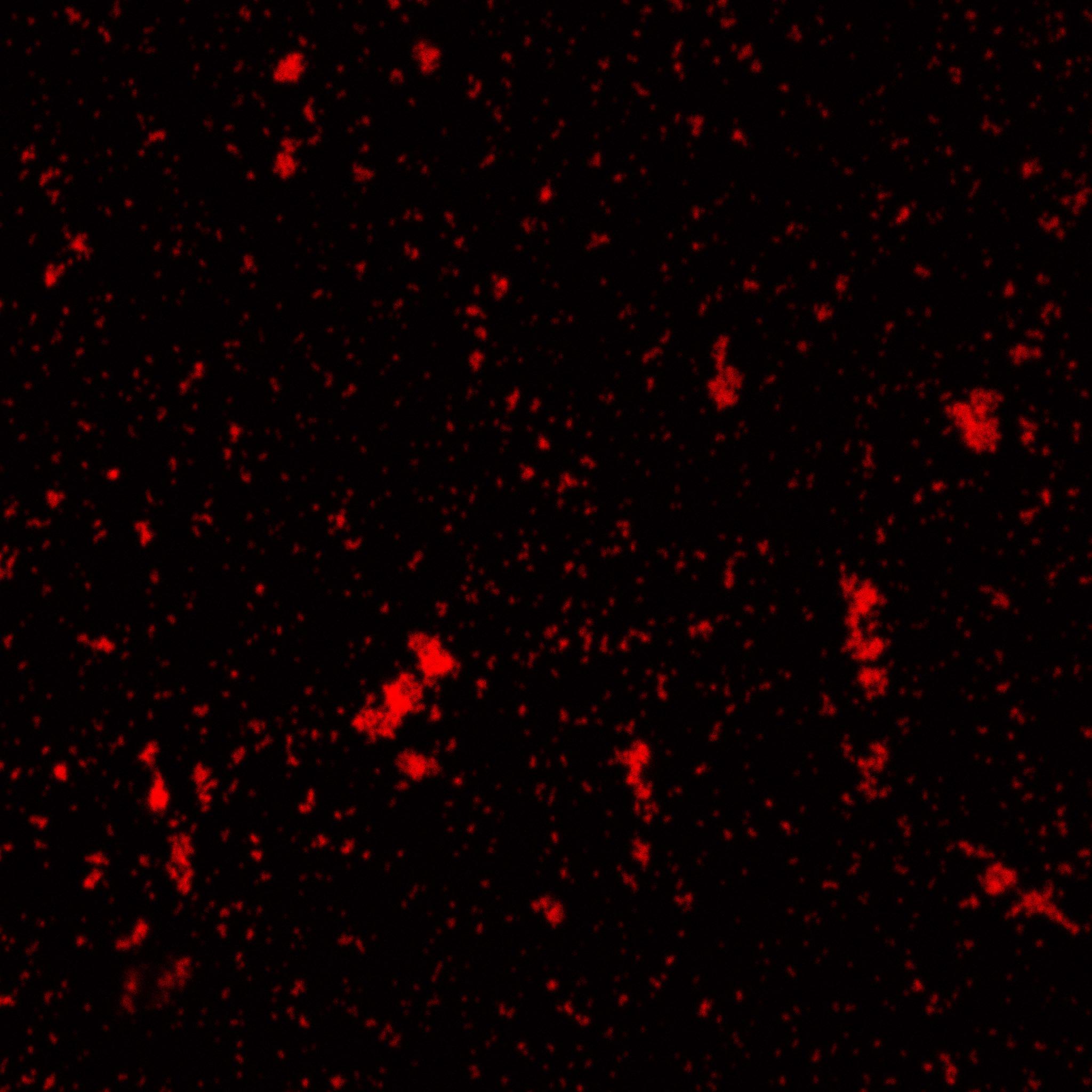
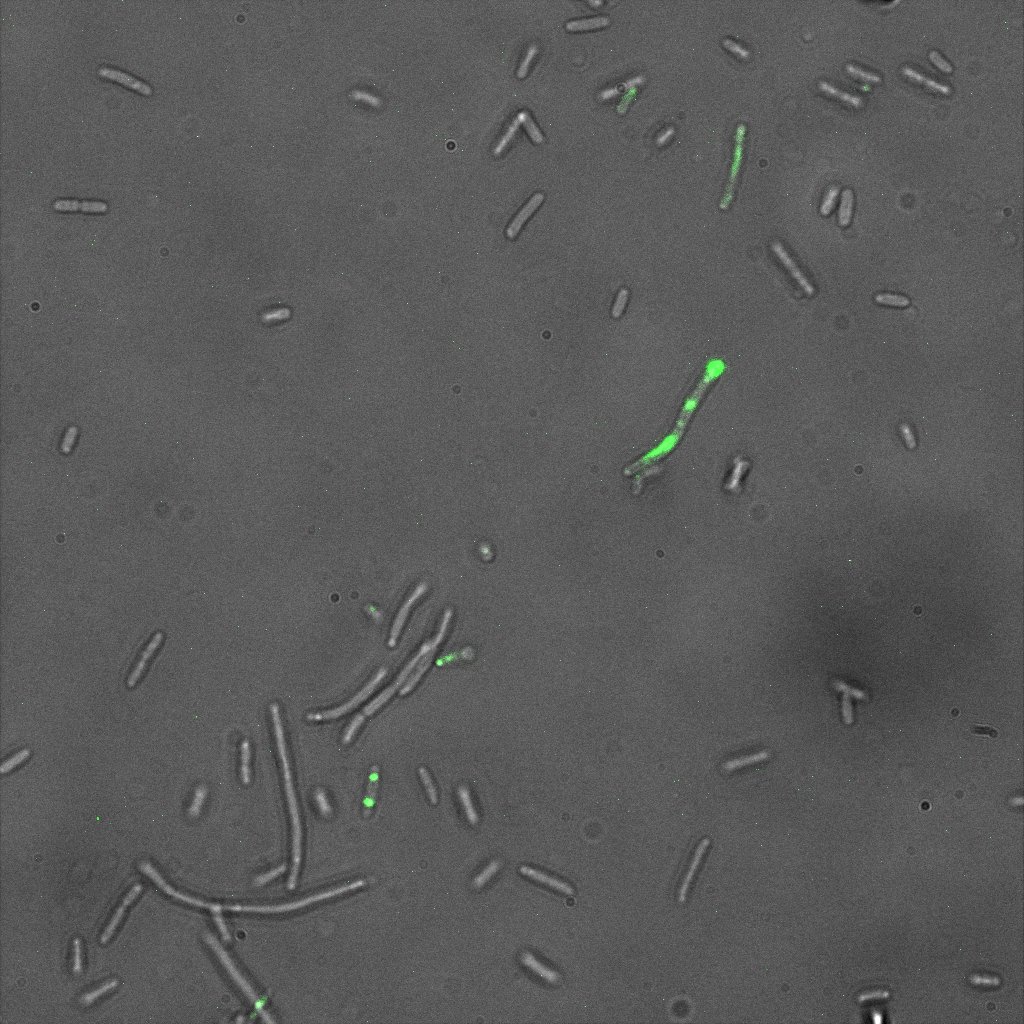
| + | [[Image:Team-EPF-Lausanne_results_confocal_positive.jpg|thumb|200px|left| Figure 3: Confocal microscopy image showing outer CY5 labeling of the nanoparticles. The size of those nanoparticles is around 200 nm, which corresponds to the previous DLS characterization.]] |
| - | The Gibson assemblies went fine and the results matched 100% with the expected sequences. | + | |
| - | Fluorescence measurements were not really relevant since a lot of cells died in acidic pH, supposed to activate the promoter. However, even if we were not able to prove that acidic pH triggers expression of GFP, fluorescence could be seen, thus the promoters are active. Constitutive promoter showed nice results, which allowed us to deliver it in the kit (see outreach -> kit).
| + | |
| - | <br>
| + | |
| | | | |
| - | For triggering expression of degradating enzymes, arabinose sensitive promoter was chosed. The enzymes to insert were MMP9, MMP2 and gelE, the three degrading gelatin. The part BBa_I746908 was the backbone consisting in GFP driven by pBad promoter. The enzymes to insert would be either put instead of GFP or in addition to GFP. All final plasmids would have a His-tag to purify it easily.
| + | [[Image:Team-EPF-Lausanne_results_NPs_FITC.jpg|thumb|240px|left| Figure 4: Fluorescent microscopy of the FITC-Dextran loaded nanoparticles. In contrast to the rGFP-loaded ones, their cargo stay inside. Those nanoparticles have been successfully characterized and biotinylated.]] |
| - | Sequences of the enzymes and the backbones were correctly PCR amplified. The Gibson were successfull for the MMP2 and gelE, but cells transformed with MMP9 didn't give any colony.
| + | |
| - | Sequencing results of the other plasmids showed a stop codon between enzymes and GFP, the reason why they didn't appear green.
| + | |
| - | However, the enzymes preceding GFP could still be expressed and an His-Tag purification was achieved since His-tag is placed before the stop codon. The different fractions were collected and analyzed on a SDS page :
| + | |
| - | <br>
| + | |
| | | | |
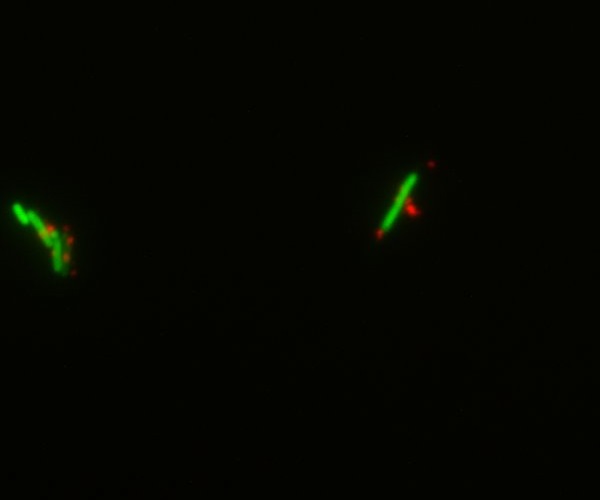
| | + | [[Image:Team-EPF-Lausanne_device1.jpg|thumb|240px|left|Figure 5: Fluorescent microscopy of GFP-expressing E.coli and nanoparticles. The bacteria were chemically labeled with streptavidin as a surrogate of the cell-surface display strategy. We then mixed them with biotinylated CY5-labeled nanoparticles. This merge image shows that the coupling is successful and that the nanoparticles are attached to the E.coli.]] |
| | | | |
| - | Streptavidin export at the outer membrane was also needed to achieve our project in order to couple nanoparticles to bacteria. Three streptavidin were used to increase the success chances. The three were amplified and inserted into the Biobrick Bba_K523013 insted of YFP. So streptavidin was fused with INP supposed to export it at the outer membrane.
| + | <br><br><br><br><br><br><br><br> |
| - | The sequencing results of the Gibson Assembly were fine and proved that streptavidin was well in frame with INP in the three cases.
| + | <br><br><br><br><br><br><br><br> |
| - | The functionnal assays were less promising. An assay was performed with antibody anti streptavidin but showed no significant results. Biotin was then used because of its smaller size and showed .... on va le refaire demain/aujourd'hui.
| + | <br><br><br><br><br><br><br><br> |
| - | A Western Blot was performed with a lot of unspecific noise, still showing some bands at the expected size of INP-streptavidin fusion (53KDa).
| + | <br><br><br><br><br><br><br><br> |
| - | <br> | + | <br><br><br> |
| | | | |
| - | We also spent time on improving the part used as backbone for streptavidin export. Since the initial group didn't access a good microscop, we proved the location by performing an assay with antiYFP antibody, and it was proved to co-localize with the fluorescence obtained by excitaion of YFP, proving its export to the outer membrane. | + | ==Cell surface expression:== |
| | + | |
| | + | '''Characterization of an existing part:''' |
| | + | The part BBa_K523013 (INP-YFP construct to export YFP at the membrane) had been characterized only by a comparison of pellet and supernatant fluorescence after centrifugation. <br>We wanted to characterize it better to be sure that it was expressed on the outer membrane. We successfully showed that the INP_YFP fusion protein was expressed at the membrane. |
| | + | |
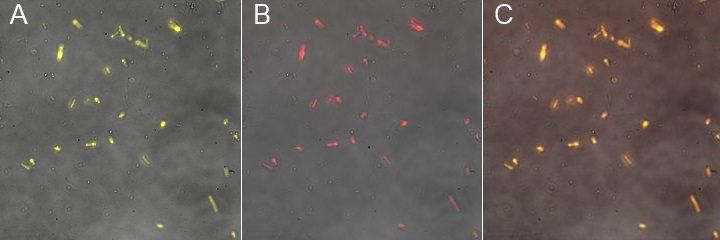
| | + | This fluorescence microscopy image shows that YFP direct excitation signal colocalizes with YFP-antibody signal, meaning that the protein is not only at the membrane, but at the outer one. |
| | + | [[Image:Team-EPF-Lausanne_INP-WFP-merged-3pics.jpg|thumb|700px|left| Figure 6: INP-YFP expressing cells A)detection by WFP excitation (514nm); B) detection by biotinylated anti-YFP antibody and avidin daylight (650nm); C) Merged images show colocalization, proving that the fusion protein is expressed at the outer membrane.]] |
| | + | <br><br><br><br><br><br><br><br><br><br><br><br> |
| | + | |
| | + | '''Expression of streptavidin at the cell surface:''' |
| | + | Gibson assemblies of the three different streptavidin constructs worked and sequencing results matched with what expected. The growth curve of transformed E.Coli showed delayed growth, but bacteria still divide with an acceptable rate. The assay with a fluorescent biotin supposed to bind streptavidin gave some positive results (some bacteria appeared fluorescent when excited at the corresponding wavelength) but since the negative control also showed fluorescence, nothing could be proved. However, a Western blot against streptavidin showed bands at the expected size around 50 kDa of streptavidin, proving that it was expressed. |
| | + | |
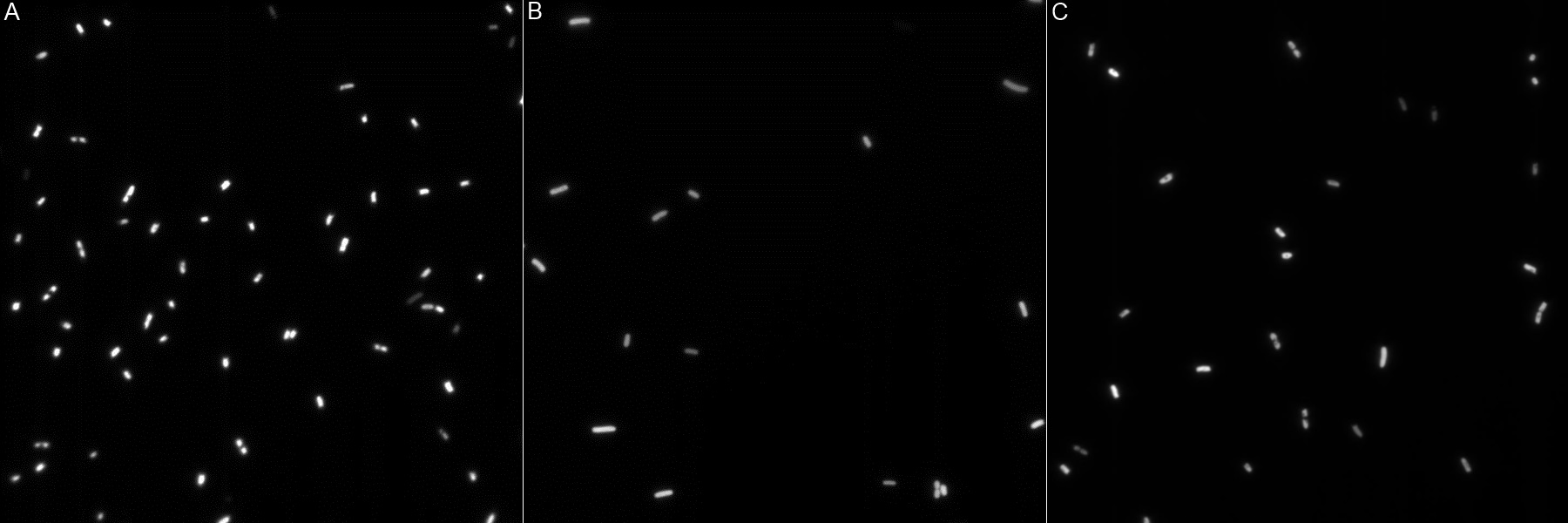
| | + | [[Image:Team-EPF-Lausanne_ISA5_biotin_3.10(RGB).jpg|thumb|300px|left| Figure 7: INP-streptavidin expressing cells from INP-strepta alive construct. Detection was made using fluorescently labeled biotin (green). Note that some cells were also positive on the negative control (competent cells), though they were less numerous.]] |
| | + | [[Image:Team-EPF-Lausanne_WB1.jpg|thumb|375px|left| Figure 8: Western blot made from total protein of INP-strepta (from all three constructs) transformed cells. Though there is much unspecific noise, there are bands at the right size.]] |
| | + | |
| | + | <br><br><br><br><br><br><br><br><br><br><br><br><br> |
| | + | <br><br><br><br><br><br><br> |
| | + | |
| | + | ==Sensing:== |
| | + | We inserted two pH-dependent and one constitutive promoter in front of a superfolded GFP sequence ( [http://parts.igem.org/Part:BBa_I746908 Biobrick BBa_I746916, Main page] ). All three promoters as well as the respective backbones were successfully isolated and amplified by PCR. |
| | + | The Gibson assemblies also worked and the sequencing results showed a 100% match between the inserted promoters and the reference sequence.<BR> |
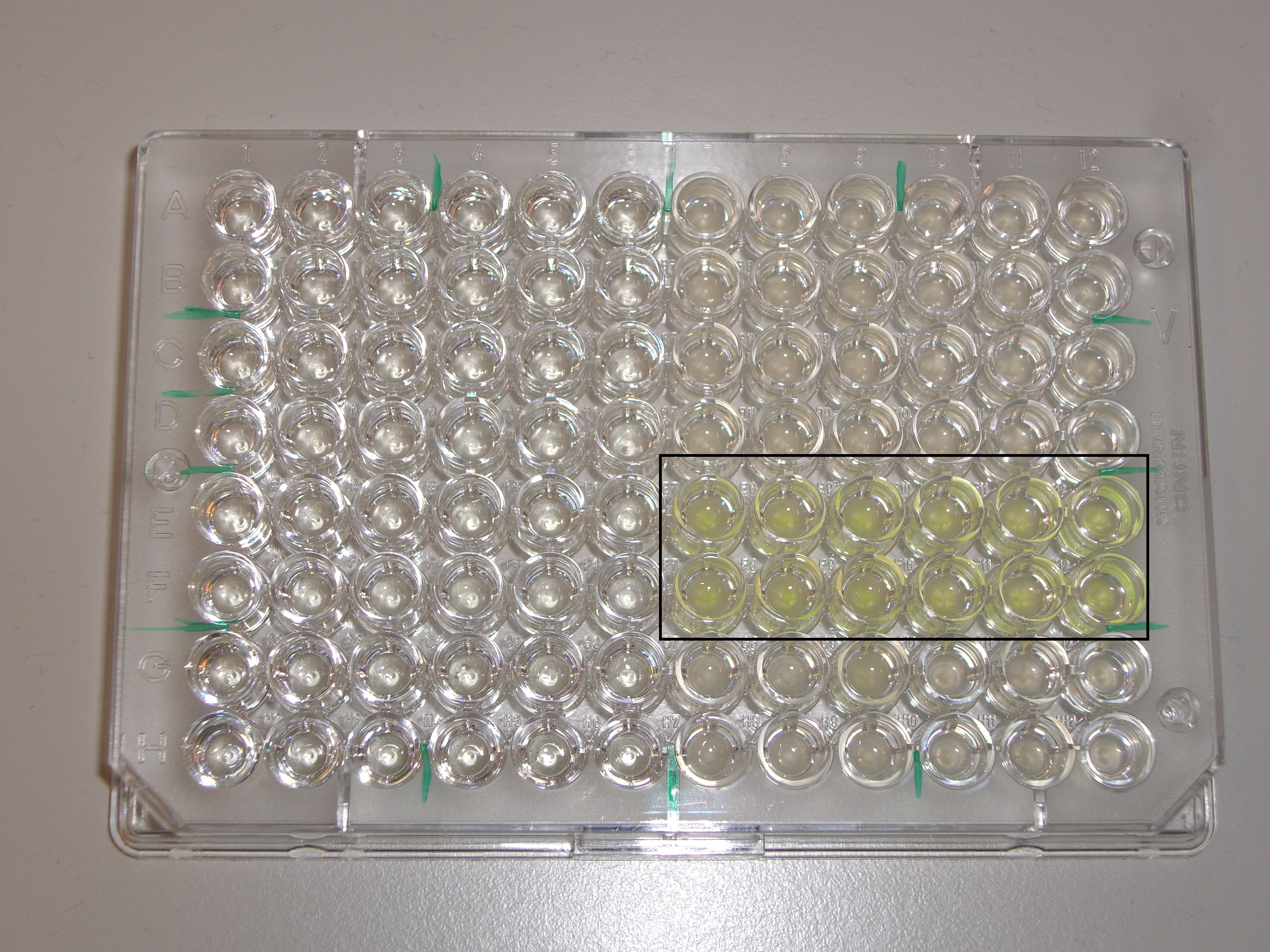
| | + | Fluorescence measurements with the Plate Reader were not conclusive since a lot of cells died in acidic pH, supposed to activate the promoter. However, even if we were not able to prove that acidic pH triggers expression of GFP, fluorescence could be seen under the microscope. This indicated that the promoters are functional. The constitutive promoter worked as expected, inducing the expression of superfolded GFP strongly. We used the fact that those cells' fluorescence could be seen by the naked eye and put the plasmid in our human practice kit ([https://2013.igem.org/Team:EPF_Lausanne/Kit Our Kit ]). |
| | + | [[Image: Team-EPFL-Lausanne PCR_1.1+1.2+1.3_BB.jpg|thumb|200px|left|Figure 9: 0.8% Gel, PCRs of the three backbones ]] |
| | + | [[Image: Team-EPFL-Lausanne PlateReader.jpg|thumb|500px|right|Figure 10: Plate used for the Plate Reader experiment. The cell marked in a black rectangle are the ones containing the constitutive promoter.]]<br><br><br><br><br> |
| | + | |
| | + | |
| | + | <br><br><br><br><br><br><br><br><br><br><br><br><br> |
| | + | [[Image: Team-EPFL-Lausanne 1.3_MS_WATER-MOPS-HEPES.jpg|thumb|700px|center|Figure 11 :FRAC_image, exposure: 400ms, Multiplier 1, from left to right: pH: 7, pH: 6.5, pH: 8.5 ]] |
| | <br> | | <br> |
| | | | |
| - | In conclusion, we can say that except minor problems, cloning succedeed well. The Gibson assemblies globally worked out and we had no trouble growing the resulting transformed bacteria. The most delicate part was the characterization of our parts with functionnal assays. However, a lot of parts showed encouraging results but would maybe need to be studied in more detail. The naoparticles is the part that worked out well, nanoparticles could be synthetized and loaded. This project was ambitious and was almost achieved, and we are really proud of sharing our results with the iGEM community!
| + | ==Effector:== |
| | + | With the exception of MMP9, the Gibson Assemblies worked for both Gelatinase GelE and MMP2. The primers that were designed had introduced a stop codon upstream of GFP. But since we had planned to do constructs with and without GFP attached to the gelatinase, we continued our experiments. The Western Blot with an anti-His tag antibody did not work. The His-Tag may be hidden in the protein, contain a mutation or could simply not bind to the nickel columns as they were stored improperly. |
| | + | <br> |
| | | | |
| | + | ==Overall:== |
| | + | Globally, we can say that our cloning was successful. Most of the Gibson assemblies worked and we had no particular troubles growing the resulting transformed bacteria. The most delicate part was the characterization of our parts with functional assays. Though some experiments didn't allow us to make conclusions, a lot of parts showed encouraging results. They would maybe need to be studied in more detail for further improvement. The nanoparticle module worked very well. Our positive results would allow to try to load real drugs in a next nanoparticles batch. |
| | + | This project was ambitious and was almost achieved, and we are really proud to share our experience with the iGEM community! |
| | | | |
| | | | |
| | {{Template:EPFL2013Footer}} | | {{Template:EPFL2013Footer}} |
 "
"