Team:SDU-Denmark/Tour31
From 2013.igem.org
| (36 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
<html> | <html> | ||
<h2>Specifications</h2> | <h2>Specifications</h2> | ||
| - | <h4>Rewiring | + | <h4>Rewiring the <span class="specialWord">E. coli</span> machinery</h4> |
<p> | <p> | ||
| - | <span class="intro">As mentioned, the nonpathogenic E. coli</span> strain MG1655 only lacks the prenyltransferase enzyme from the rubber tree before it is able to produce natural rubber. | + | <span class="intro">As mentioned, the nonpathogenic <span class="specialWord">E. coli</span></span> strain MG1655 only lacks the prenyltransferase enzyme from the rubber tree before it is able to produce natural rubber. Apart from introducing the HRT2 gene to the strain, we also wanted to enhance the IPP production of the cell for further rubber production. As a consequence of these goals, we studied the prenyltransferase and the MEP pathway, which subsequently enabled us to reach our rubber-producing ambitions. |
</p><p> | </p><p> | ||
| - | <a class=" | + | <a class="imageGallery alignRight" style="width:240px" target="_blank" href="https://static.igem.org/mediawiki/2013/f/fb/SDU2013_Samlet_MEP_pathway_V.1.2.png" title="The MEP pathway"> |
<img src="https://static.igem.org/mediawiki/2013/f/fb/SDU2013_Samlet_MEP_pathway_V.1.2.png" style="width:240px" /> | <img src="https://static.igem.org/mediawiki/2013/f/fb/SDU2013_Samlet_MEP_pathway_V.1.2.png" style="width:240px" /> | ||
| - | The MEP pathway | + | The MEP pathway - Will open in a new tab due to the size. Edited from Alexander Steinbüchel: Production of rubber-like polymers by microorganism, Current Opinion in Microbiology 2003. 6:261-270. |
</a> | </a> | ||
</p> | </p> | ||
<p><span class="intro">MEP pathway</span><br> | <p><span class="intro">MEP pathway</span><br> | ||
| - | The biosynthesis of isoprenoids in bacteria works through the <span class="tooltipLink">MEP pathway.</span> <span class="tooltip"> | + | The biosynthesis of isoprenoids in bacteria works through the <span class="tooltipLink">MEP pathway.</span> <span class="tooltip"><span class="tooltipHeader">MEP pathway</span>methylerythritol phosphate pathway</span> |
| + | <ul> | ||
| + | <li> | ||
| - | </ | + | The pathway is initiated with a condensation reaction between <span class="tooltipLink">GAP</span> <span class="tooltip"><span class="tooltipHeader">GAP</span>D-glyceraldehyde-3-phosphate</span> and pyruvate to produce <span class="tooltipLink">DXP</span> <span class="tooltip"><span class="tooltipHeader">DXP</span>1-deoxy-D-xylulose-5-phosphate</span> and CO<sub>2</sub> catalysed by <span class="tooltipLink">Dxs.</span> <span class="tooltip"><span class="tooltipHeader">DXS</span>1-deoxyxylulose-5-phosphate synthase</span> |
| - | < | + | </li><li> |
| + | |||
| + | DXP is reductively isomerized to <span class="tooltipLink">MEP</span> <span class="tooltip"><span class="tooltipHeader">MEP</span>2-C-methyl-D-erythritol-4-phosphate</span> by <span class="tooltipLink">Dxr</span> <span class="tooltip"><span class="tooltipHeader">Dxr</span>1-deoxy-D-xylulose-5-phosphate reductoisomerase</span> in a reversible NADPH-dependent reaction | ||
| + | |||
| + | </li><li> | ||
| + | |||
| + | This is subsequently converted to <span class="tooltipLink">CDP-ME</span> <span class="tooltip"><span class="tooltipHeader">CDP-ME</span>4-diphosphocytidyl-2-C-methyl-D-erythritol</span> in a CTP substrate inhibited reaction catalysed by <span class="tooltipLink">IspD.</span> <span class="tooltip"><span class="tooltipHeader">IspD</span>4-diphosphocytidyl-2-C-methyl-D-erythritol synthase</span> | ||
| - | |||
DXP is not only a substrate in the isoprenoids biosynthesis, but also for the reversible synthesis of B<sub>1</sub>-vitamin and further synthesis of B<sub>6</sub>-vitamin in the thiamine biosynthesis pathway. | DXP is not only a substrate in the isoprenoids biosynthesis, but also for the reversible synthesis of B<sub>1</sub>-vitamin and further synthesis of B<sub>6</sub>-vitamin in the thiamine biosynthesis pathway. | ||
| - | </ | + | </li><li> |
| - | The next step in the MEP pathway is an ATP-dependent phosphorylation of the C<sub>2</sub> hydroxyl group of CDP-ME, giving <span class="tooltipLink">CDP-MEP</span> <span class="tooltip">4-diphosphocytidyl-2-C-methyl-D-erythritol-2-phosphate</span> catalysed by <span class="tooltipLink">IspE.</span> <span class="tooltip">4-diphosphocytidyl-2-C-methyl-D-erythritol kinase</span> | + | The next step in the MEP pathway is an ATP-dependent phosphorylation of the C<sub>2</sub> hydroxyl group of CDP-ME, giving <span class="tooltipLink">CDP-MEP</span> <span class="tooltip"><span class="tooltipHeader">CDP-MEP</span>4-diphosphocytidyl-2-C-methyl-D-erythritol-2-phosphate</span> catalysed by <span class="tooltipLink">IspE.</span> <span class="tooltip"><span class="tooltipHeader">IspE</span>4-diphosphocytidyl-2-C-methyl-D-erythritol kinase</span> |
| - | </ | + | </li><li> |
| + | |||
| + | CDP-MEP is cyclized to <span class="tooltipLink">MEcPP</span> <span class="tooltip"><span class="tooltipHeader">MEcPP</span>2-C-methyl-D-erythritol-2,4-cyclodiphosphat</span> by <span class="tooltipLink">IspF</span> <span class="tooltip"><span class="tooltipHeader">IspF</span>2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase</span> and in the next reaction <span class="tooltipLink">IspG</span> <span class="tooltip"><span class="tooltipHeader">IspG</span>1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate synthase</span> catalyzes the ring opening of the cyclic pyrophosphate and subsequent C3-reductive dehydration of MEcPP to <span class="tooltipLink">HMBPP.</span> <span class="tooltip"><span class="tooltipHeader">HMBPP</span>1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate</span> | ||
| + | |||
| + | </li><li> | ||
| - | + | The MEP pathway’s final step, the conversion of HMBPP to <span class="tooltipLink">IPP</span> <span class="tooltip"><span class="tooltipHeader">IPP</span>isopentenyl diphosphate</span> and | |
| + | <span class="tooltipLink">DMAPP</span> <span class="tooltip"><span class="tooltipHeader">DMAPP</span>dimethylallyl diphosphate</span>, is catalysed by <span class="tooltipLink">IspH.</span> <span class="tooltip"><span class="tooltipHeader">IspH</span>4-hydroxyl-3-methyl-butenyl-1-disphosphate reductase</span> | ||
| + | </li> | ||
| + | </ul> | ||
</p><p> | </p><p> | ||
| - | + | There exists a natural equilibrium between the two isomers IPP and DMAPP. The enzyme <span class="tooltipLink">Idi</span> <span class="tooltip"><span class="tooltipHeader">Idi</span>isopentenyl diphosphat isomerase</span> catalyses the reversible isomerization reaction between IPP and DMAPP in order to balance the ratio of IPP and DMAPP according to the cellular demands under various | |
| - | + | ||
<span class="sourceReference">conditions.</span> | <span class="sourceReference">conditions.</span> | ||
<span class="tooltip"> | <span class="tooltip"> | ||
<span class="tooltipHeader">Source:</span> | <span class="tooltipHeader">Source:</span> | ||
| - | + | Zhao L, Chang WC, Xiao Y, Liu HW, Liu P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu Rev Biochem. 2013;82:497-530. doi: 10.1146/annurev-biochem-052010-100934. | |
| + | |||
| + | <a href="http://www.ncbi.nlm.nih.gov/pubmed/23746261" target="_blank">(Link)</a> | ||
</span> | </span> | ||
| + | </p><p> | ||
In our system this will cause dislocation of the equilibrium towards IPP production, since IPP is the five carbon building block in the rubber biosynthesis. In accordance, the reversible formation of B<sub>1</sub>-vitamin from DXP will be dislocated towards the production of DXP rather than B<sub>1</sub>-vitamin (See our model). | In our system this will cause dislocation of the equilibrium towards IPP production, since IPP is the five carbon building block in the rubber biosynthesis. In accordance, the reversible formation of B<sub>1</sub>-vitamin from DXP will be dislocated towards the production of DXP rather than B<sub>1</sub>-vitamin (See our model). | ||
| Line 48: | Line 64: | ||
<br> | <br> | ||
| - | <a class="popupImg alignLeft" style="width:240px" target="_blank" href="https://static.igem.org/mediawiki/2013/0/07/SDU2013_Gummi_syntesen_V.1.1.png" title=" | + | <a class="popupImg alignLeft" style="width:240px" target="_blank" href="https://static.igem.org/mediawiki/2013/0/07/SDU2013_Gummi_syntesen_V.1.1.png" title="Illustration of the rubber synthesis by the prenyltransferase (HRT2) - Rubber is polymerized from a DMAPP primer by IPP extension via prenyltransferase(HRT2). Each new IPP extents the rubber molecule by 5 carbon. Edited from Alexander Steinbüchel: Production of rubber-like polymers by microorganism, Current Opinion in Microbiology 2003. 6:261-270."> |
<img src="https://static.igem.org/mediawiki/2013/7/7a/SDU2013_Small_Gummi_syntesen_V.1.1.png" style="width:240px" /> | <img src="https://static.igem.org/mediawiki/2013/7/7a/SDU2013_Small_Gummi_syntesen_V.1.1.png" style="width:240px" /> | ||
| - | + | An illustration of the rubber synthesis. | |
</a> | </a> | ||
</p> | </p> | ||
| + | |||
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | |||
<p> | <p> | ||
<span class="intro">Polyprenyltransferase</span><br> | <span class="intro">Polyprenyltransferase</span><br> | ||
| - | When used for rubber production in E. coli, the MEP pathway is followed by a prenyltransferase catalysed rubber polymerisation reaction, where one IPP molecule condenses with DMAPP followed by condensation products: | + | When used for rubber production in <span class="specialWord">E. coli</span>, the MEP pathway is followed by a prenyltransferase catalysed rubber polymerisation reaction, where one IPP molecule condenses with DMAPP followed by condensation products: |
| - | <span class="tooltipLink">GPP</span> <span class="tooltip">geranyl diphosphate, C<sub>10</sub></span>, | + | <span class="tooltipLink">GPP</span> <span class="tooltip"><span class="tooltipHeader">GPP</span>geranyl diphosphate, C<sub>10</sub></span>, |
| - | <span class="tooltipLink">FPP</span> <span class="tooltip">farnesyl diphosphate, C<sub>15</sub></span> and | + | <span class="tooltipLink">FPP</span> <span class="tooltip"><span class="tooltipHeader">FPP</span>farnesyl diphosphate, C<sub>15</sub></span> and |
| - | <span class="tooltipLink">GGPP | + | <span class="tooltipLink">GGPP</span> etc.<span class="tooltip"><span class="tooltipHeader">GGPP</span>geranylgeranyl diphosphate, C<sub>20</sub></span> |
| - | In Hevea brasiliensis, the rubber polymerisation reaction is catalysed by a cis-1,4-polyprenyltransferase known as | + | In <span class="specialWord">Hevea brasiliensis</span>, the rubber polymerisation reaction is catalysed by a cis-1,4-polyprenyltransferase known as |
<span class="sourceReference">Hevea Rubber Transferase 2 (HRT2).</span> | <span class="sourceReference">Hevea Rubber Transferase 2 (HRT2).</span> | ||
<span class="tooltip"> | <span class="tooltip"> | ||
<span class="tooltipHeader">Source:</span> | <span class="tooltipHeader">Source:</span> | ||
| - | + | Alexander Steinbüchel: Production of rubber-like polymers by microorganism, Current Opinion in Microbiology 2003. 6:261-270. (book) <a href="http://www.ncbi.nlm.nih.gov/pubmed/12831902" target="_blank">(Link)</a> | |
</span> | </span> | ||
</p><p> | </p><p> | ||
| + | |||
<br> | <br> | ||
<br> | <br> | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | <small> | + | <small>Note: Ecocyc noted that overexpression of Dxs results in increased isoprenoid biosynthesis, but also yields a reduced growth rate for the whole cell. <a target="_blank" href="http://www.ncbi.nlm.nih.gov/pubmed/11180061">Ecocycs reference</a></small> |
| + | |||
| + | |||
<br> | <br> | ||
Latest revision as of 18:51, 28 October 2013
Specifications
Rewiring the E. coli machinery
As mentioned, the nonpathogenic E. coli strain MG1655 only lacks the prenyltransferase enzyme from the rubber tree before it is able to produce natural rubber. Apart from introducing the HRT2 gene to the strain, we also wanted to enhance the IPP production of the cell for further rubber production. As a consequence of these goals, we studied the prenyltransferase and the MEP pathway, which subsequently enabled us to reach our rubber-producing ambitions.
MEP pathway
The biosynthesis of isoprenoids in bacteria works through the MEP pathway. MEP pathwaymethylerythritol phosphate pathway
- The pathway is initiated with a condensation reaction between GAP GAPD-glyceraldehyde-3-phosphate and pyruvate to produce DXP DXP1-deoxy-D-xylulose-5-phosphate and CO2 catalysed by Dxs. DXS1-deoxyxylulose-5-phosphate synthase
- DXP is reductively isomerized to MEP MEP2-C-methyl-D-erythritol-4-phosphate by Dxr Dxr1-deoxy-D-xylulose-5-phosphate reductoisomerase in a reversible NADPH-dependent reaction
- This is subsequently converted to CDP-ME CDP-ME4-diphosphocytidyl-2-C-methyl-D-erythritol in a CTP substrate inhibited reaction catalysed by IspD. IspD4-diphosphocytidyl-2-C-methyl-D-erythritol synthase DXP is not only a substrate in the isoprenoids biosynthesis, but also for the reversible synthesis of B1-vitamin and further synthesis of B6-vitamin in the thiamine biosynthesis pathway.
- The next step in the MEP pathway is an ATP-dependent phosphorylation of the C2 hydroxyl group of CDP-ME, giving CDP-MEP CDP-MEP4-diphosphocytidyl-2-C-methyl-D-erythritol-2-phosphate catalysed by IspE. IspE4-diphosphocytidyl-2-C-methyl-D-erythritol kinase
- CDP-MEP is cyclized to MEcPP MEcPP2-C-methyl-D-erythritol-2,4-cyclodiphosphat by IspF IspF2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase and in the next reaction IspG IspG1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate synthase catalyzes the ring opening of the cyclic pyrophosphate and subsequent C3-reductive dehydration of MEcPP to HMBPP. HMBPP1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate
- The MEP pathway’s final step, the conversion of HMBPP to IPP IPPisopentenyl diphosphate and DMAPP DMAPPdimethylallyl diphosphate, is catalysed by IspH. IspH4-hydroxyl-3-methyl-butenyl-1-disphosphate reductase
There exists a natural equilibrium between the two isomers IPP and DMAPP. The enzyme Idi Idiisopentenyl diphosphat isomerase catalyses the reversible isomerization reaction between IPP and DMAPP in order to balance the ratio of IPP and DMAPP according to the cellular demands under various conditions. Source: Zhao L, Chang WC, Xiao Y, Liu HW, Liu P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu Rev Biochem. 2013;82:497-530. doi: 10.1146/annurev-biochem-052010-100934. (Link)
In our system this will cause dislocation of the equilibrium towards IPP production, since IPP is the five carbon building block in the rubber biosynthesis. In accordance, the reversible formation of B1-vitamin from DXP will be dislocated towards the production of DXP rather than B1-vitamin (See our model).
 An illustration of the rubber synthesis.
An illustration of the rubber synthesis.
Polyprenyltransferase
When used for rubber production in E. coli, the MEP pathway is followed by a prenyltransferase catalysed rubber polymerisation reaction, where one IPP molecule condenses with DMAPP followed by condensation products:
GPP GPPgeranyl diphosphate, C10,
FPP FPPfarnesyl diphosphate, C15 and
GGPP etc.GGPPgeranylgeranyl diphosphate, C20
In Hevea brasiliensis, the rubber polymerisation reaction is catalysed by a cis-1,4-polyprenyltransferase known as
Hevea Rubber Transferase 2 (HRT2).
Source:
Alexander Steinbüchel: Production of rubber-like polymers by microorganism, Current Opinion in Microbiology 2003. 6:261-270. (book) (Link)
Note: Ecocyc noted that overexpression of Dxs results in increased isoprenoid biosynthesis, but also yields a reduced growth rate for the whole cell. Ecocycs reference
 "
"
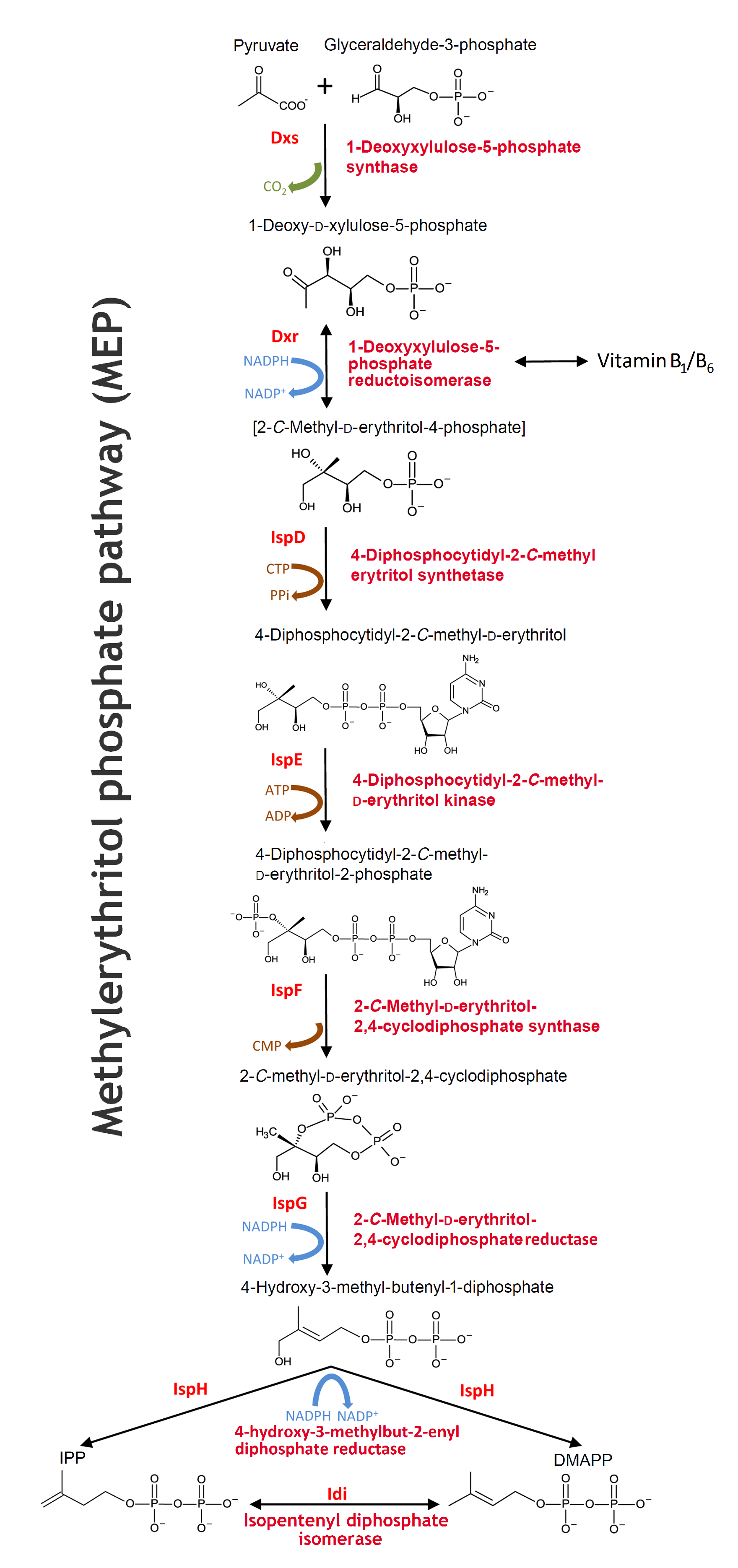 The MEP pathway - Will open in a new tab due to the size. Edited from Alexander Steinbüchel: Production of rubber-like polymers by microorganism, Current Opinion in Microbiology 2003. 6:261-270.
The MEP pathway - Will open in a new tab due to the size. Edited from Alexander Steinbüchel: Production of rubber-like polymers by microorganism, Current Opinion in Microbiology 2003. 6:261-270.