Team:TU Darmstadt/safety/Labjournal
From 2013.igem.org
(Difference between revisions)
| Line 185: | Line 185: | ||
<li>Ligate 5 µl of the reaction mix with 50 ng EcoRI/PstI resticted and purified pSB1C3 over night at 16 °C <br> For Ligation use the T4-Ligase and fresh T4-Ligase Buffer with ATP</li> | <li>Ligate 5 µl of the reaction mix with 50 ng EcoRI/PstI resticted and purified pSB1C3 over night at 16 °C <br> For Ligation use the T4-Ligase and fresh T4-Ligase Buffer with ATP</li> | ||
<li>After heat-inactivation of the ligase by 80 °C for 15 min use 2 µl of the mix for heat-shock transformation</li> | <li>After heat-inactivation of the ligase by 80 °C for 15 min use 2 µl of the mix for heat-shock transformation</li> | ||
| - | <li>Plate out the transformation in black petridishies | + | <li>Plate out the transformation in black petridishies with LB-cmp media.</li> |
</ul> | </ul> | ||
| - | + | <Br><Br><Br> | |
<font size="3" color="#F0F8FF" face="Arial regular"> | <font size="3" color="#F0F8FF" face="Arial regular"> | ||
| Line 196: | Line 196: | ||
Construction of pSB1C3-petZ | Construction of pSB1C3-petZ | ||
| - | + | <br> | |
| - | For the construction of pSB1C3-petZ we isolate the petZ gene with PCR from the gBlocks C1 and C3: | + | For the construction of pSB1C3-petZ we isolate the petZ gene with PCR from the gBlocks C1 and C3 follow this protocol: |
<br><br> | <br><br> | ||
| Line 204: | Line 204: | ||
<ul style="margin-left:50px; margin-right:50px; text-align:justify; "> | <ul style="margin-left:50px; margin-right:50px; text-align:justify; "> | ||
| - | <li>Use the Primer pair Pre-PetZ/For-PetZ and an annealing temperature of 55 °C for the isolation PCR follow the Q5 PCR | + | <li>Use the Primer pair Pre-PetZ/For-PetZ and an annealing temperature of 55 °C for the isolation PCR follow the Q5 PCR protokol from NEB Biolabs. </li> |
| + | <li> After 30 cycles, use 5 µl for the control gelelectrophoreses and clean the rest of the reaktion with the Wizard SV Gel and PCR Clean-Up System from Promega.</li> | ||
| + | <li> Restict 500 ng of the PCR product and 1 µg of pSB1C3 with EcoRI and PstI (10 U each) at 37 °C for 30 min. </li> | ||
| + | <li> Perform the ligation with an molar ratio of 3:1 (insert:vector).<br> For Ligation use the T4-Ligase and fresh T4-Ligase Buffer with ATP</li> | ||
| + | <li> Transfrom 2 µl of the ligation mix into <i>E. coli</i> DH5alpha. </li> | ||
| + | <Li> Plate out the transformation of LB-cmp media.</li> | ||
</ul> | </ul> | ||
Revision as of 12:05, 29 September 2013
Labjournal
Assembly of the gBlocks
Based on the pDawn Plasmid we desinged the light induced kill switch and synthesized the construct as gBlock Fragments on IDT. We assembeled the 10 Fragments follow the Protocol:
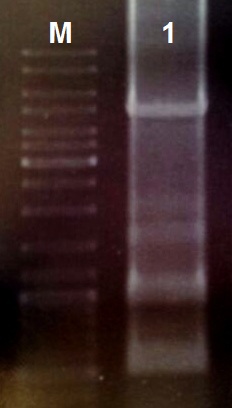
- Reconstitute the gBlock Fragments in 10 µl TE Buffer
- Use 1 µl of each gBlock Fragment for a PCR with the Q5 Polymerase
- Perform the PCR Reaktion with the Primers Prefix and Suffix and an annealing temperature of 55 °C
(30 cycles) - Take 5 µl of the 50 µl reaction for DNA-gelelectrophoreses
- Restrict the other 45 µl with the restrictionenzymes EcoRI and PstI (10 U each) for 1 h at 37 °C
- Ligate 5 µl of the reaction mix with 50 ng EcoRI/PstI resticted and purified pSB1C3 over night at 16 °C
For Ligation use the T4-Ligase and fresh T4-Ligase Buffer with ATP - After heat-inactivation of the ligase by 80 °C for 15 min use 2 µl of the mix for heat-shock transformation
- Plate out the transformation in black petridishies with LB-cmp media.
Construction of pSB1C3-petZ
For the construction of pSB1C3-petZ we isolate the petZ gene with PCR from the gBlocks C1 and C3 follow this protocol:
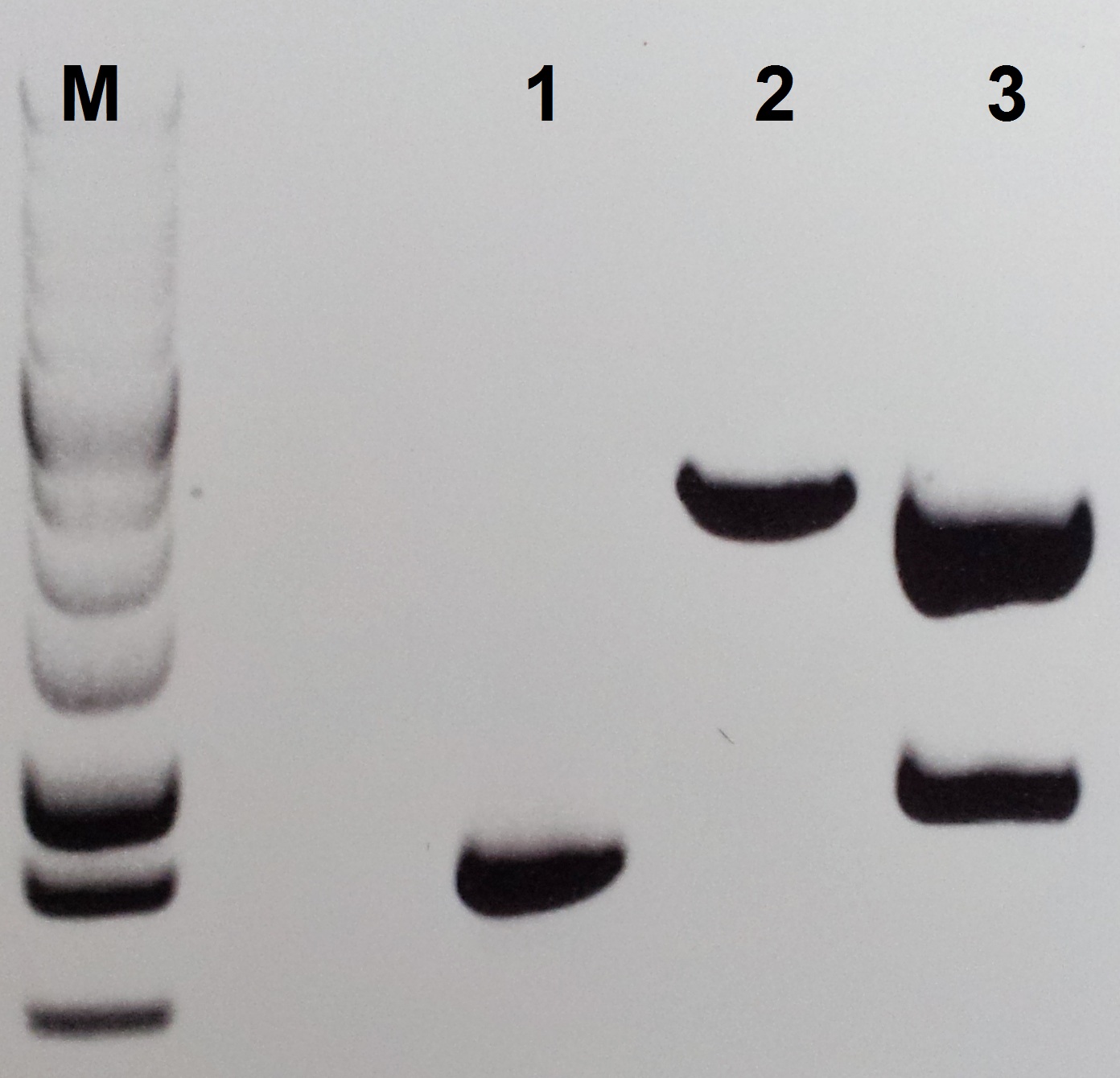
- Use the Primer pair Pre-PetZ/For-PetZ and an annealing temperature of 55 °C for the isolation PCR follow the Q5 PCR protokol from NEB Biolabs.
- After 30 cycles, use 5 µl for the control gelelectrophoreses and clean the rest of the reaktion with the Wizard SV Gel and PCR Clean-Up System from Promega.
- Restict 500 ng of the PCR product and 1 µg of pSB1C3 with EcoRI and PstI (10 U each) at 37 °C for 30 min.
- Perform the ligation with an molar ratio of 3:1 (insert:vector).
For Ligation use the T4-Ligase and fresh T4-Ligase Buffer with ATP - Transfrom 2 µl of the ligation mix into E. coli DH5alpha.
- Plate out the transformation of LB-cmp media.
 "
"