Team:BYU Provo/Notebook/LargePhage/Springexp/Period4/Dailylog
From 2013.igem.org
| Large Phage May - June Notebook: June 10 - June 30 Daily Log
| ||
|
|
6/10/13 Today we looked at a paper describing efficient ways to propagate large phage. They suggested agarose instead of agar, and at a concentration of 0.15% which is 1.5 g/L. Dr. Grose explained to us that agarose is more stable and forms larger pores, requiring a smaller amount of powder to produce a solid, stable gel. LB Agarose top agar can be plated onto normal LB bottom agar. We will make up some agarose top agar and see what size of plaques we get. Wednesday we will need to start an overnight culture in a 250 mL flask (using 25 mL of LB broth). We will a piece of a small colony and put it in the flask along with 20 ug of adenine per mL. It will incubate on the shaker until class starts.
6/12/13 Results from 6/10/13 Unfortunately none of our agarose media set up, even in the jars that were left on the counter. This was unfortunate because it meant our seven plates were unreadable. We will need to make a higher concentration of agarose to learn what it takes for agarose top agar to solidify on LB agar plates.
We let one flask incubate on a shaker for ___ hours (until _:__ pm) and the other incubate on a shaker for ___ hours (until _:__ pm). The bacteria was pelleted and the phage-containing supernatant was transferred to a new test tube and refridgerated at +4C. KS and BDM
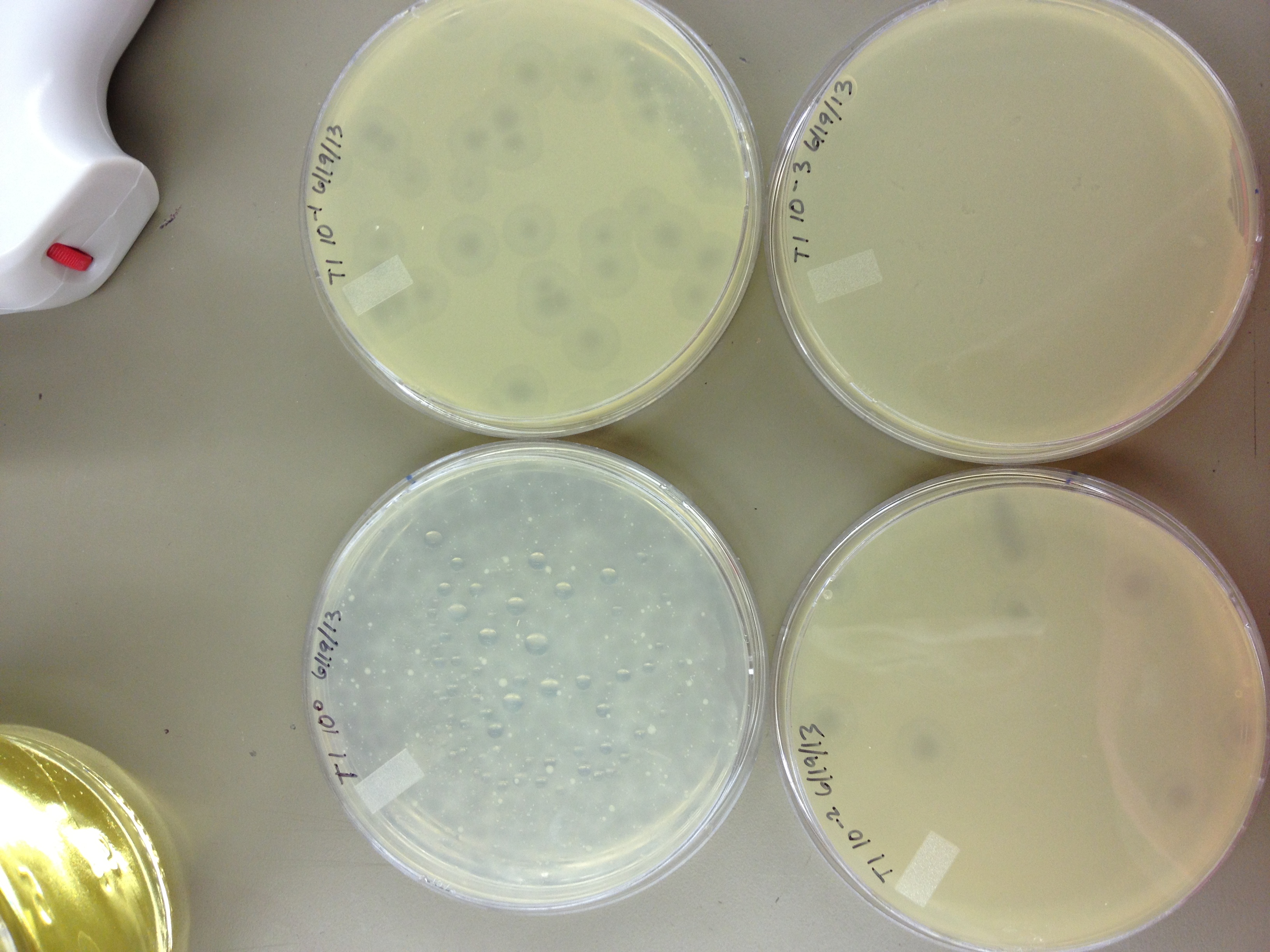
6/14/13 Our mutagenesis liquid cultures were not removed from the flask on Wednesday night; instead, they were pelleted on Thursday morning. We will test the titer soon to see if it worked and didn't get diluted or destroyed. Today, we will: Change top agar concentrations so they will solidify Test plaque sizes on new top agar Do a dilution series on the T1 and mutagenized phage
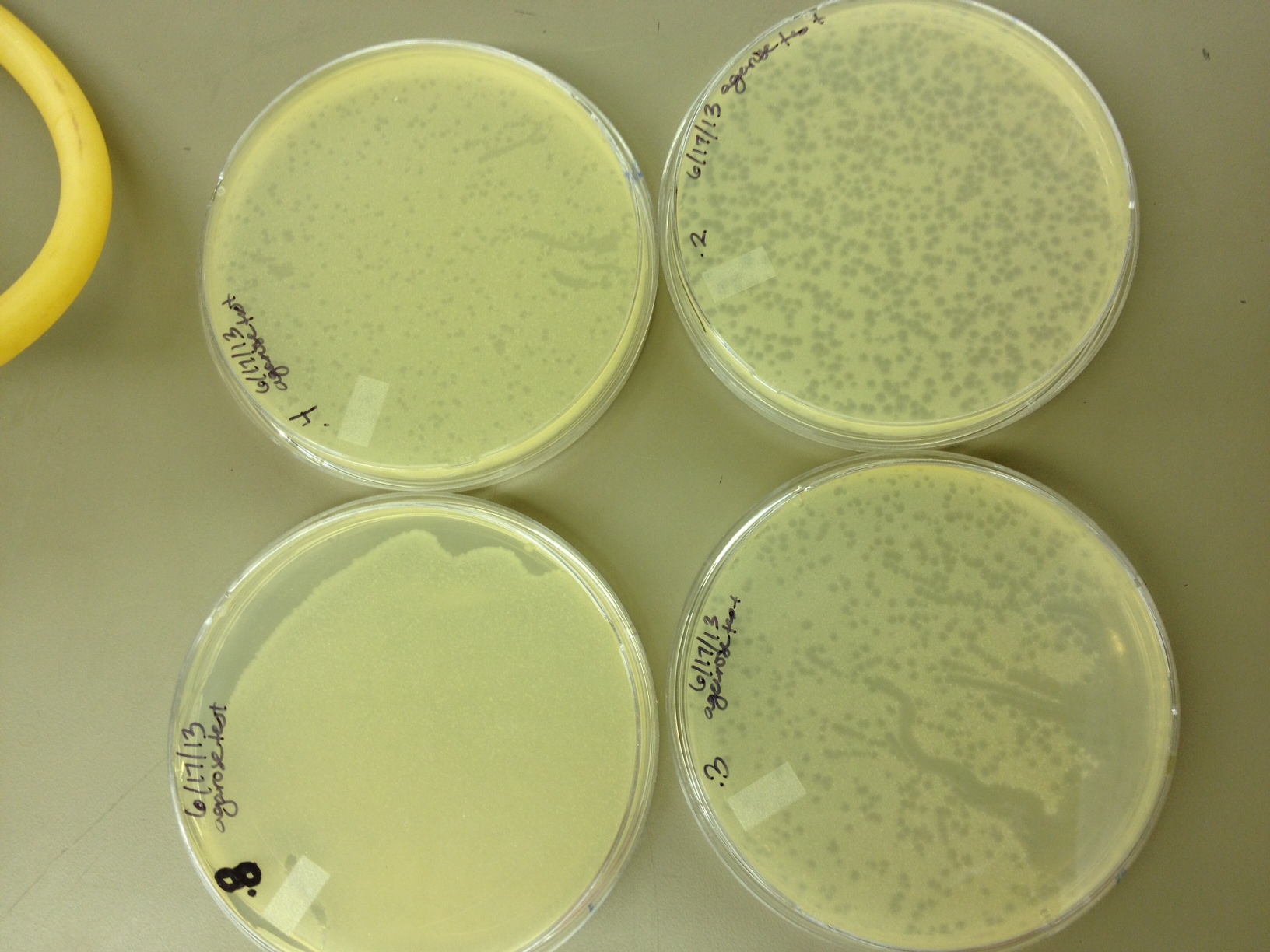
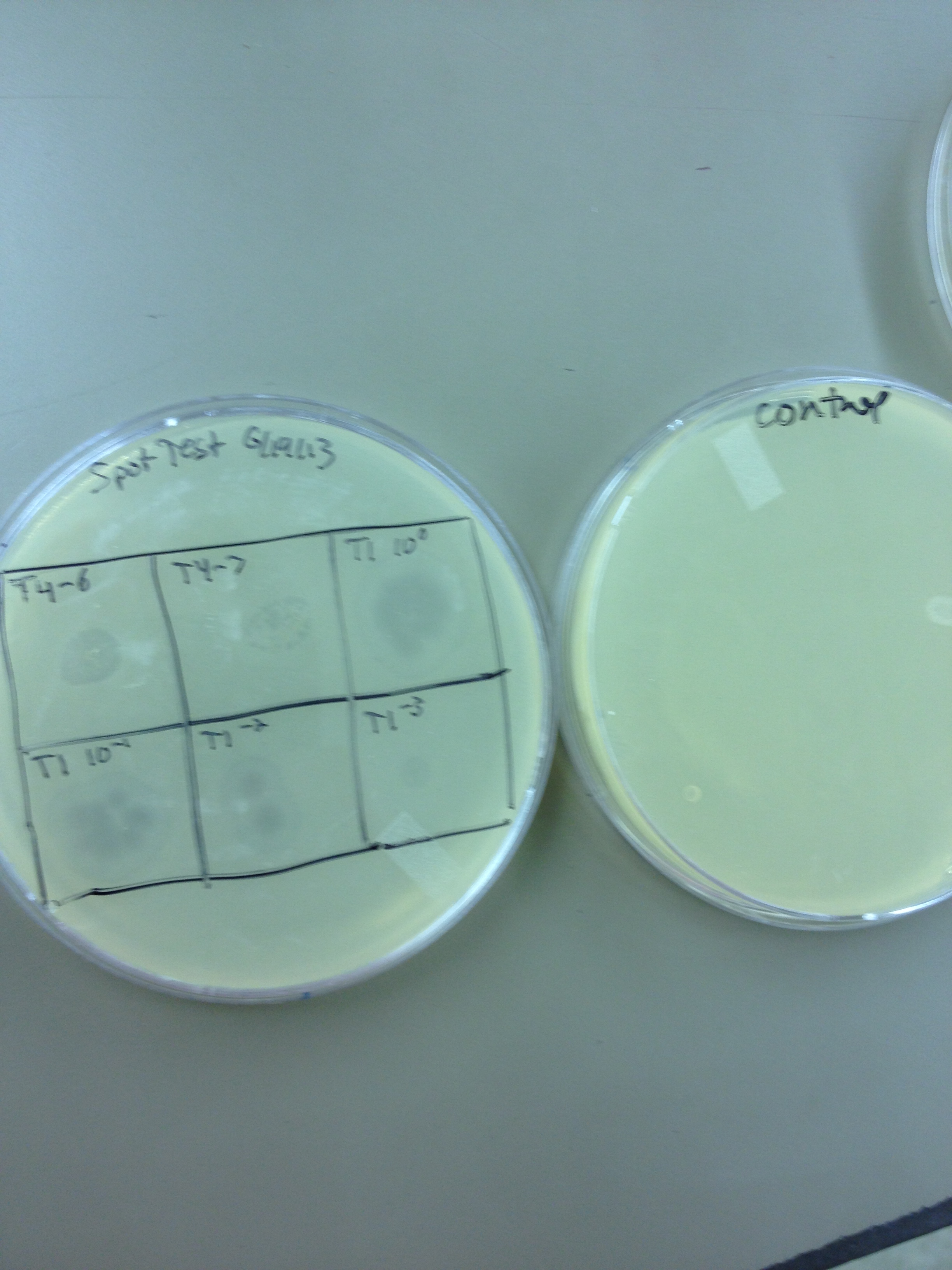
6/17/13 Today we did our agarose plaque size test. We took 2 uL from our T4 stock diluted to 10^-6 and infected 0.5 mL of E. coli B. We made seven identical test tubes, and plated each in varying concentrations of agarose and broth. We tested .8% agarose, .4%, .3%, .2%, .15%, .1%, and .075%. These will incubate overnight at 37C and we will measure the change in plaque sizes as the concentration of agarose decreases. Also, we spot-tested our T1 dilution series (10^-3 to 10^-11) on a plate of E. coli B to make sure it infects. If it works ok, we will plate out each dilution level so we can get a good estimate of the PFUs in the T1 stock and start mass producing T1 for later steps in our mutagenesis procedure.
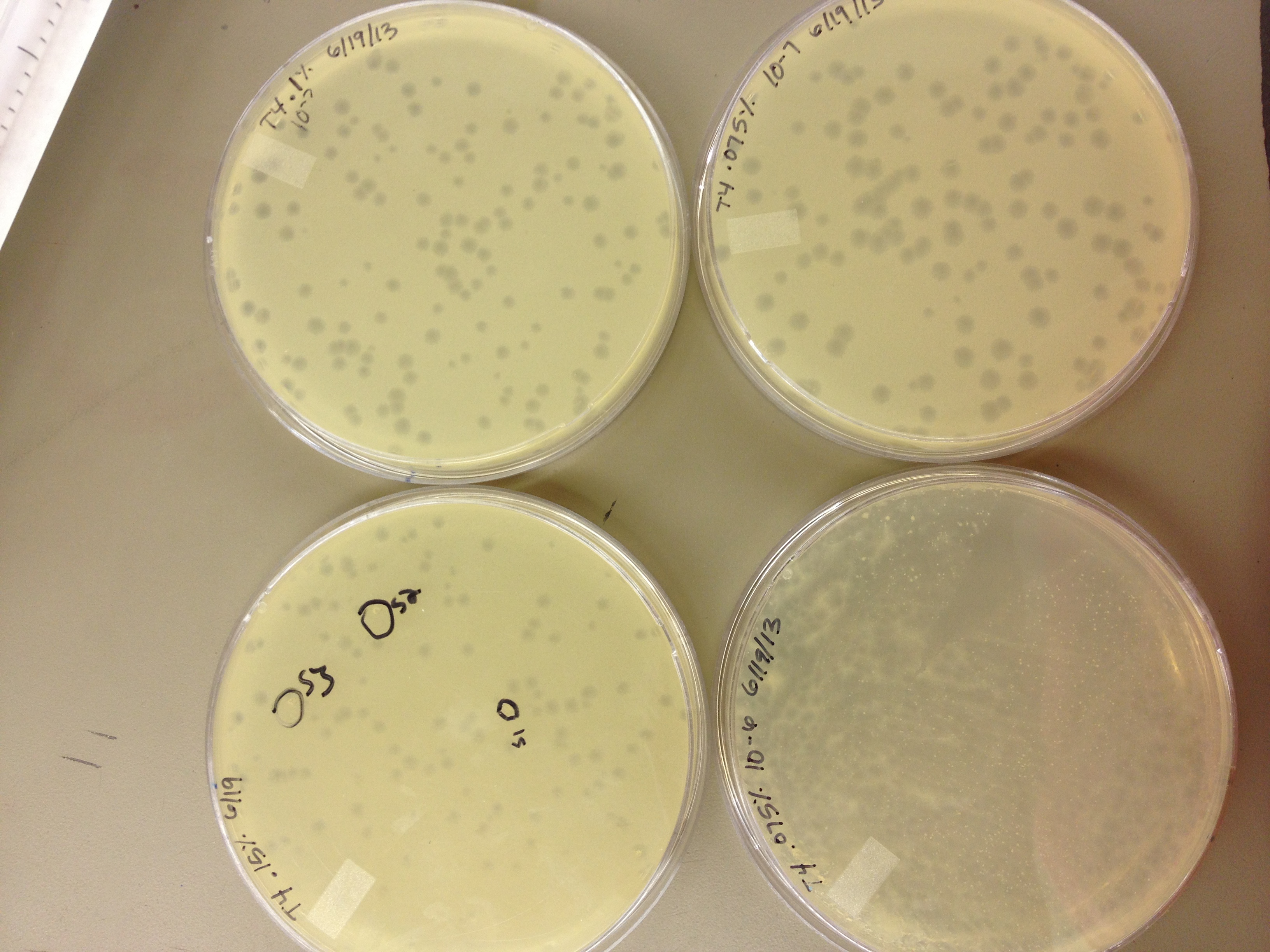
6/19/13 Results from 6/17/2013 - We found that our T1 stock is at a very low titer. We only saw clearage on our spot test at 10^-3. There will have to be a lot done to grow it up to a high titer. Our agarose test worked very well. There were no visible plaques on 0.8% agarose, and the plaques increased in size down to 0.15% agarose. Our titer was too high, because at 0.075% we got web plates and couldn't accurately measure the diameter of the plaques. We had no trouble getting the agarose to solidify at .075% On 6/18/2013, we repeated the lower level of the agarose test by testing .15%, .1%, .075%, .055%, .035%, and .015%. We want to know the lowest concentration at which agarose will still solidify and show us visible plaque diameters which we can measure. When we see small mutant plaques at this low level of agarose concentration, we will know that they are larger-than-normal phage. We also infected a plate with 10uL of 10^-3 T1 phage to see if it made a web, plaques, or cleared.
6/21/13
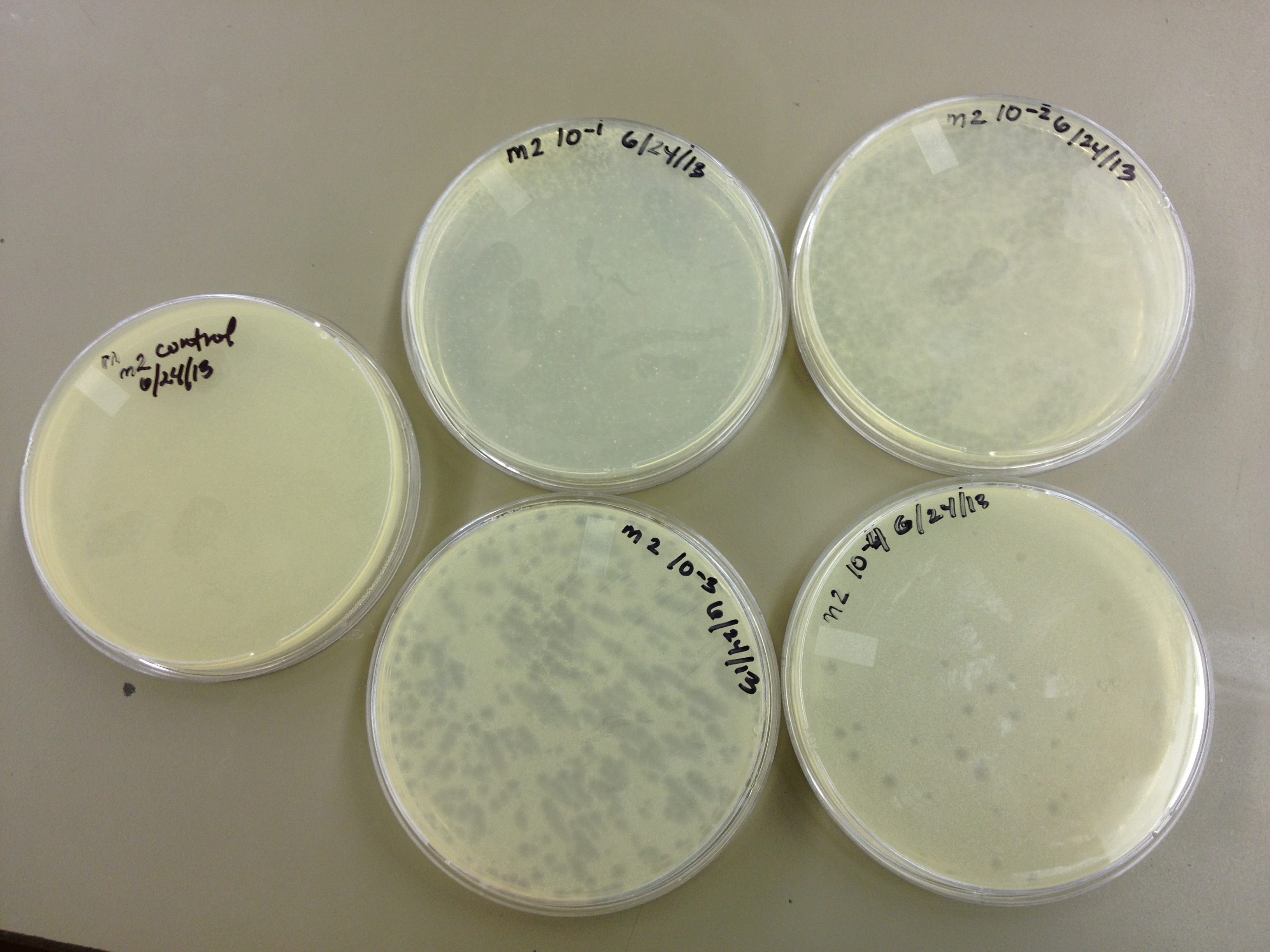
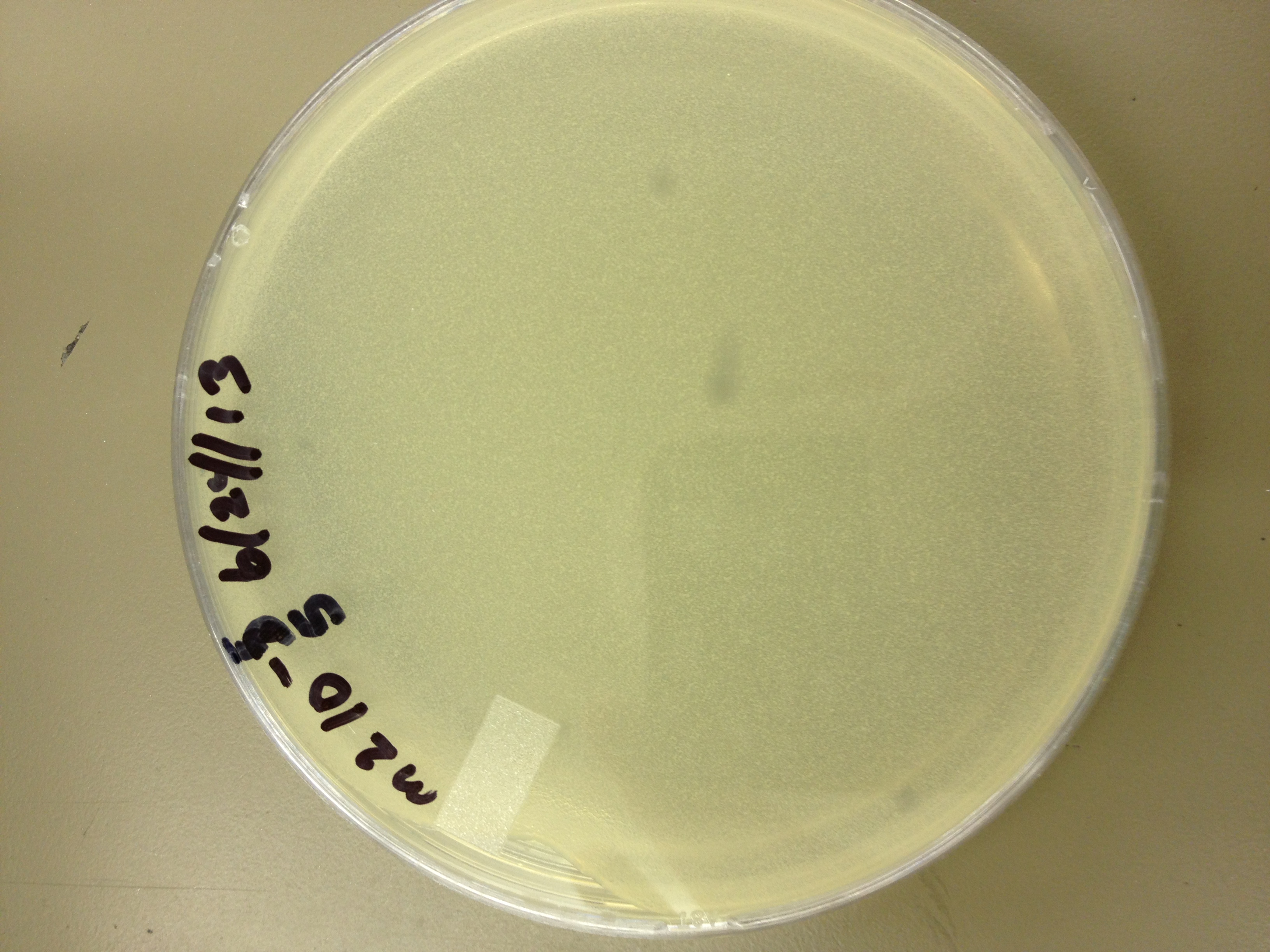
6/24/13 Today we did a titer on the "M2" phage (The second round of mutagenesis)
6/26/13 Results for the M2 titer. Plaques go down to 10^-5 (about 6 plaques) So our concentration is somewhere between 10^-5 and 10^-6.
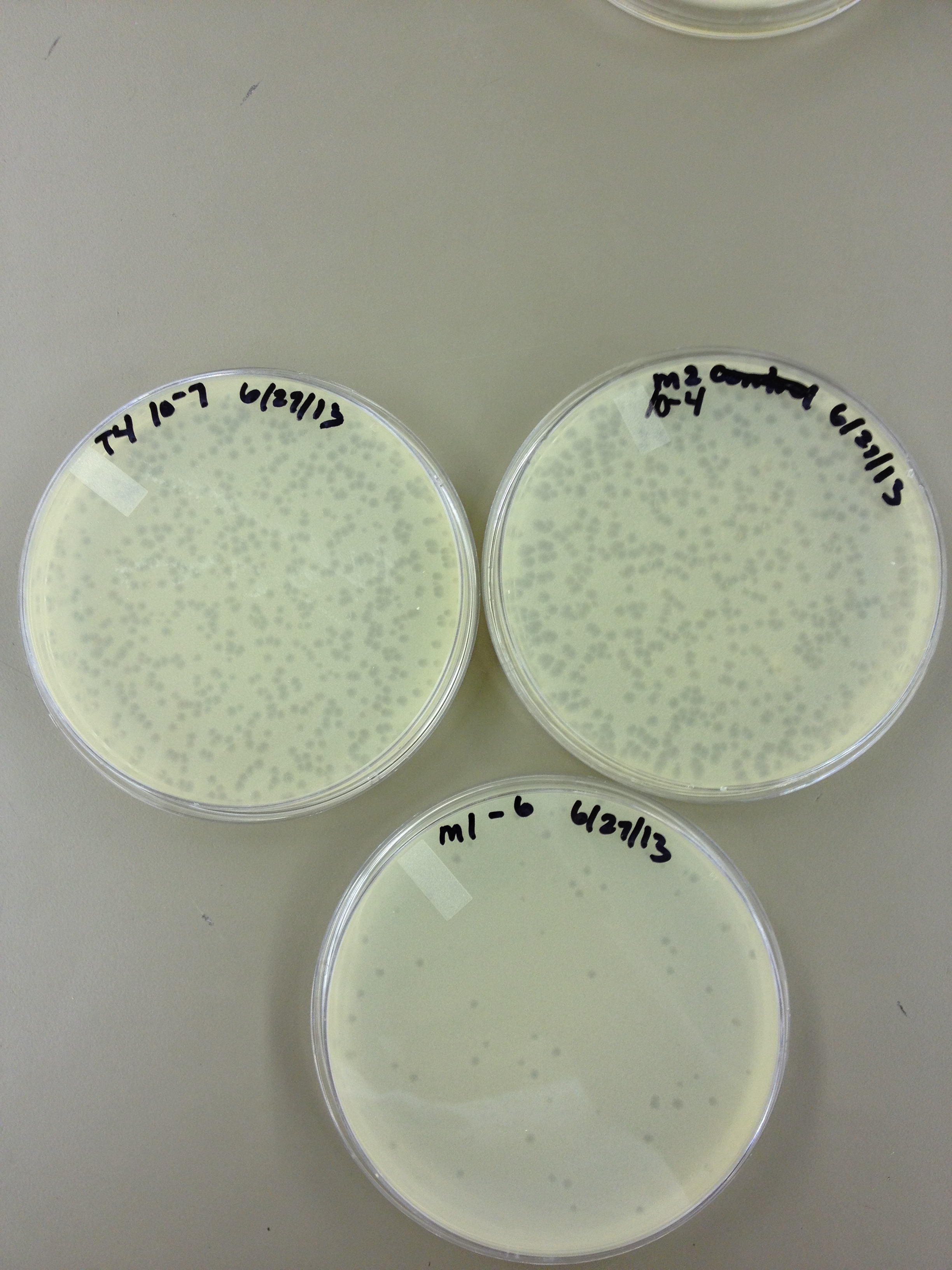
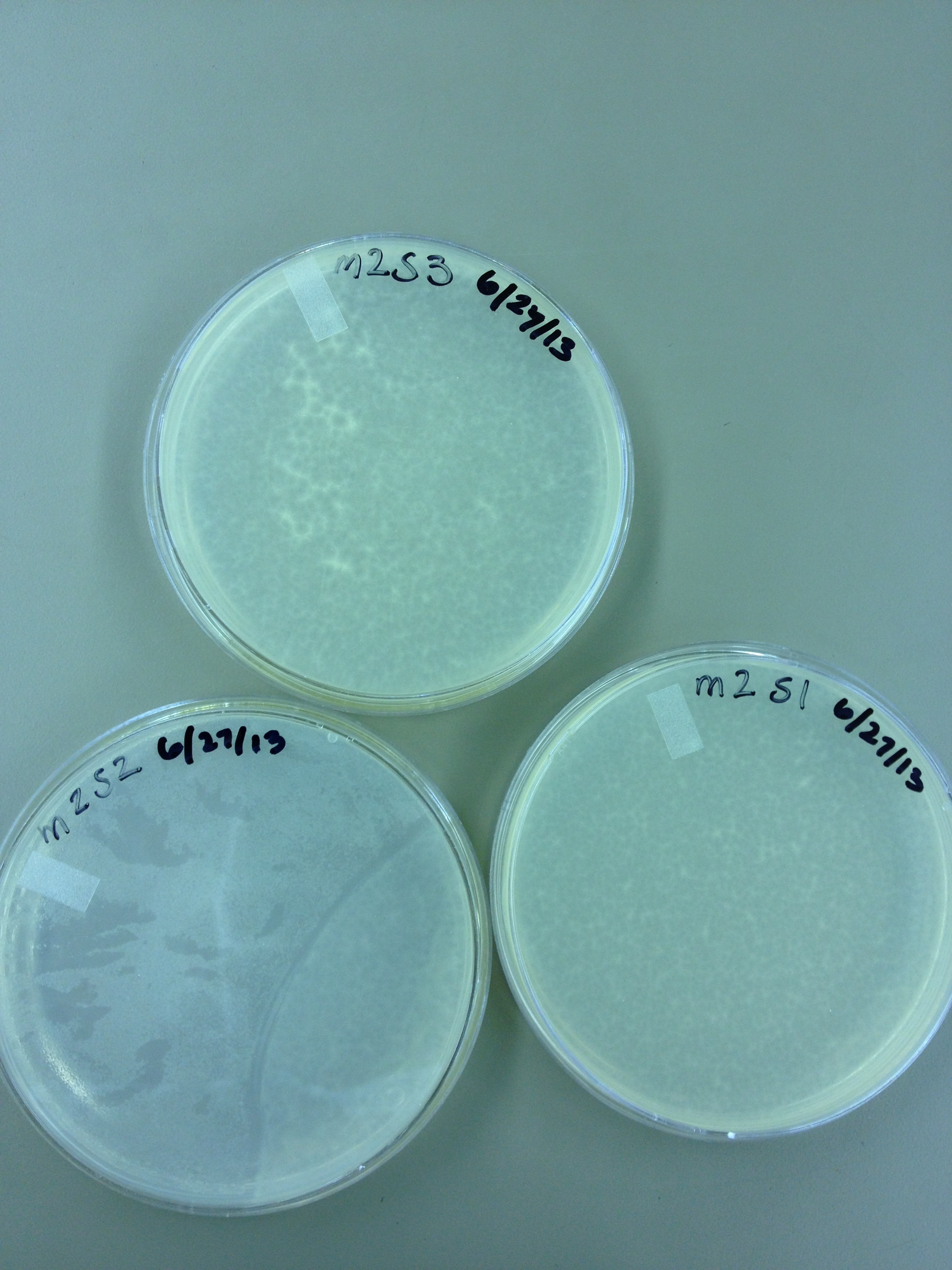
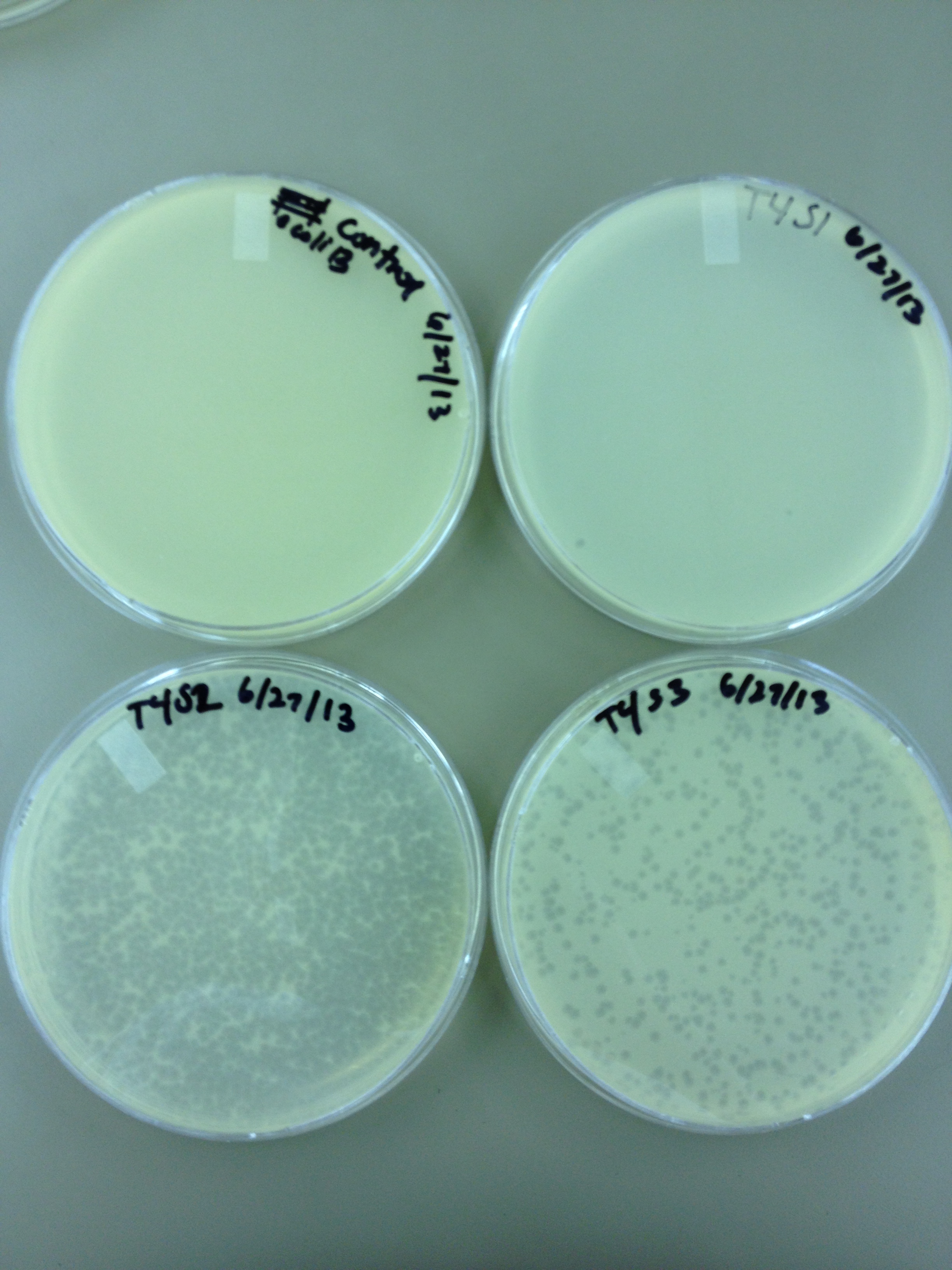
6/27/13 Today we did another round of mutagenesis using the same protocol. We also did spot tests on a few of the pin prick plaques found from the experiment on 6/24.
6/28/13 Here are the results from yesterday's experiments. We are slightly concerned because in general the plaques are smaller so it's tough to see true mutants.
|
|
 "
"