Team:Edinburgh/Human Practices/Waste Treatment/Metal toxicity
From 2013.igem.org
Hristianita (Talk | contribs) |
Hristianita (Talk | contribs) |
||
| (12 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<div class='content'> | <div class='content'> | ||
| - | <h3> | + | <h3> Metal toxicity </h3> |
| - | + | ==Biotoxicity== | |
| - | + | ||
| - | + | ||
| - | + | Heavy metal cause poisoning effect on human body by interacting with the normal biochemistry in metabolic process. They are converted to their stable oxidation state (ion) in the stomach after ingested and combine with proteins and enzymes etc. to form strong chemical bonds. | |
| - | The function of the enzyme is therefore inhibited by the poisoning metal, whereas the protein-metal complex | + | The function of the enzyme is therefore inhibited by the poisoning metal, whereas the protein-metal complex reacts with a metabolic enzyme as a substrate. The reaction scheme below indicates that enzyme (E) and substrate(S) react in either the lock-and-key or the induced fit pattern. In either case, a substrate combines with an enzyme in a highly specific way to form an activated enzyme-substrate complex (ES*). |
| - | [[File:Metals_equation1..png]] | + | <span style="text-align: center">[[File:Metals_equation1..png|link=]]</span> |
| - | An enzyme cannot interact with other | + | An enzyme cannot interact with other substrates until it is freed (state E). In multi-enzyme complex (consisting of 3 or more enzymes) which includes enzymes for an entire sequence, reaction between the product from one enzyme and a second one takes place in a chain process. The final enzyme yields the final product as indicated in the equation below. |
| - | [[File:Metals_equation2..png]] | + | <span style="text-align: center">[[File:Metals_equation2..png|link=]]</span> |
| - | The final produce F inhibits further actions by reacting with E1 since it does not exist at the beginning of the reaction. Hence, E1 is not able to | + | The final produce F inhibits further actions by reacting with E1 since it does not exist at the beginning of the reaction. Hence, E1 is not able to interact with any other substrate until F is utilized by the human body. If the body is not capable of utilizing the heavy metal –protein substrate, the related enzyme will be permanently blocked (F and E1 respectively), which then is not able to initiate any bio-reaction or function. Therefore, heavy metal remains in the tissue and results in bio-dysfunctions of various gravities. |
Moreover, a metal ion can easily replace the metal ion in the body’s metallo-enzyme, if they have the similar size. For example, Zn2+ in some dehydrogenating enzyme can be conveniently replaced by Cd 2+, leading to Cadmium toxicity. The most toxic form of metals in the most stable oxidation states (Cd 2+ and Pb 2+ etc.). In the most stable oxidation states, they can form very stable biotoxic compound, which is very difficult to be dissociated from metal-biomolecule compound. | Moreover, a metal ion can easily replace the metal ion in the body’s metallo-enzyme, if they have the similar size. For example, Zn2+ in some dehydrogenating enzyme can be conveniently replaced by Cd 2+, leading to Cadmium toxicity. The most toxic form of metals in the most stable oxidation states (Cd 2+ and Pb 2+ etc.). In the most stable oxidation states, they can form very stable biotoxic compound, which is very difficult to be dissociated from metal-biomolecule compound. | ||
| - | + | == Human exposure == | |
| - | [[File:Metals2..png]] | + | [[File:Metals2..png|link=]] |
| - | Humans are directly exposed to heavy | + | Humans are directly exposed to heavy metal by inhalation of metal dust and gas (Pb), as well as intake of metal-contaminated water (Cd and Hg). However, protection from metal dust and contaminated water is not sufficient to avoid exposures to heavy metals. |
| - | + | ||
| + | Industries including mining, papermaking, leather and electroplating etc., release contaminated water to the environment. Soils (including agricultural soil) are therefore polluted by rain or irrigation water where heavy metals are dissolved. The metals are taken up by plants and accumulate in their tissues. Heavy metals will also accumulate in bodies of animals if they are grazed by contaminated plant or raised in water body that are contaminated by metal ions. Humans are therefore indirectly exposed to heavy metals by consuming contaminated plants or animal. In summary, all living organisms within a given ecosystem are contaminated to some extent according to their position in the food chain. | ||
| - | < | + | <h2>Metal poisoning </h2> |
| - | < | + | <h4> Cadmium </h4> |
| - | + | ||
| - | + | According to the final report Heavy Metal in Waste published by Europe Commission in 2002, Cadmium accumulates in human body (especially in kidney) and leads to dysfunction of the kidney with impaired reabsorption of, for example, proteins, glucose and amino acids. Furthermore, skeletal damages (e.g. osteomalacia and osteoporosis etc.) are believed to be a critical effect of cadmium exposure indicated by studies on both humans and animal. | |
| - | + | ||
| - | + | Other effects of cadmium exposure are disturbances in calcium metabolism, hypercalciuria and formation of renal stones. | |
| - | + | The most notorious cadmium poisoning case is Itai-itai disease, starting around 1912 in Japan. It was caused by the considerable amount of Cadmium released in the rivers (Jinzu River) by mining companies and a mass population was seriously affected by the disease. People were exposed to cadmium through drinking water, eating contaminated rice and vegetables grown on polluted soil. Those who were affected suffered from osteomalacia (softening of the bones) and osteoporosis (loss of bone mass and weakness). Fractures were more common as the bone weakens. In extremely serious cases, Cadmium poisoning led to death after causing a wide range of damage on human body. | |
| - | The most notorious cadmium poisoning case is Itai-itai disease, starting around 1912. It was caused by the considerable amount of Cadmium released in the rivers (Jinzu River) by mining companies and a mass population was seriously affected by the disease. People were exposed to cadmium through drinking water, eating contaminated rice and vegetables grown on polluted soil.Those who were affected | + | |
Also earlier this year (June, 2013), it was reported that rice tainted with Cadmium is discovered in southern China, leading to swell in China rice import. The news indicates that heavy metal contamination (in water and soil) is still an issue in many countries, especially developing countries such India and China, where economic development outweighs environmental protection. | Also earlier this year (June, 2013), it was reported that rice tainted with Cadmium is discovered in southern China, leading to swell in China rice import. The news indicates that heavy metal contamination (in water and soil) is still an issue in many countries, especially developing countries such India and China, where economic development outweighs environmental protection. | ||
| - | < | + | <h4>Clinical effect of other metal poisoning on humans </h4> |
| - | {| | + | {|border="1" cellpadding="8" |
|- | |- | ||
| Cr | | Cr | ||
|1.Irritating respiratory effects, possible circulatory effects, effects on stomach and blood , liver and kidney effects, and increased risk of death from lung cancer | |1.Irritating respiratory effects, possible circulatory effects, effects on stomach and blood , liver and kidney effects, and increased risk of death from lung cancer | ||
| - | 2.Allergic responses (e.g., asthma and dermatitis) in sensitised individuals | + | 2. Allergic responses (e.g., asthma and dermatitis) in sensitised individuals |
|- | |- | ||
|Fe | |Fe | ||
|1. aggravates the risks of diabetes, liver cancer, heart disease and arthritis | |1. aggravates the risks of diabetes, liver cancer, heart disease and arthritis | ||
| - | 2.enlarged liver, skin pigmentation, joint diseases, loss of body hair, amenorrhea and impotence | + | 2. enlarged liver, skin pigmentation, joint diseases, loss of body hair, amenorrhea and impotence |
|- | |- | ||
|Mn | |Mn | ||
| - | |1.Long-term exposure to excessive level leads to iron-deficiency anemia | + | |1. Long-term exposure to excessive level leads to iron-deficiency anemia |
2. Increased manganese intake impairs the activity of copper metallo-enzymes | 2. Increased manganese intake impairs the activity of copper metallo-enzymes | ||
3. Parkinson like symptoms | 3. Parkinson like symptoms | ||
| Line 62: | Line 57: | ||
|- | |- | ||
|Pb | |Pb | ||
| - | |1.Biological effects depending on the level and duration of exposure. Effects may range from inhibition of enzymes to the production of marked morphological changes and death | + | |1. Biological effects depending on the level and duration of exposure. Effects may range from inhibition of enzymes to the production of marked morphological changes and death |
| - | 2.Effect on the central nervous system. Epidemiological studies suggest that low level exposure of the foetus and developing child may lead to reprotoxic effects, i.e. damage to the learning capacity and the neuropsychological development | + | 2. Effect on the central nervous system. Epidemiological studies suggest that low level exposure of the foetus and developing child may lead to reprotoxic effects, i.e. damage to the learning capacity and the neuropsychological development |
3. Effects on haemoglobin synthesis and anaemia observed in children at lead blood levels above 40 µg/dl | 3. Effects on haemoglobin synthesis and anaemia observed in children at lead blood levels above 40 µg/dl | ||
4. Kidney damages | 4. Kidney damages | ||
| Line 70: | Line 65: | ||
|1. Similar sign of illness as Cr | |1. Similar sign of illness as Cr | ||
2. Cause system dysfunctions that result in impairment of growth and reproduction | 2. Cause system dysfunctions that result in impairment of growth and reproduction | ||
| + | |} | ||
| + | |||
| - | < | + | <h2>Drinking water quality guidelines</h2> |
Access to safe drinking-water is important as a health and development issue at a national, regional and local level. WHO has published a guideline, which includes the provisional maximum concentration of metal ion in drinking water. The following table demonstrates the guideline value for different heavy metal. | Access to safe drinking-water is important as a health and development issue at a national, regional and local level. WHO has published a guideline, which includes the provisional maximum concentration of metal ion in drinking water. The following table demonstrates the guideline value for different heavy metal. | ||
| - | {| | + | {| border="1" cellpadding="8" |
|- | |- | ||
|Element | |Element | ||
| Line 97: | Line 94: | ||
|0.01mg/litre | |0.01mg/litre | ||
|} | |} | ||
| + | |||
| + | |||
| + | <h2> References </h2> | ||
| + | |||
| + | 1.European Commission. 2002. Heavy metal in waste. URL: http://ec.europa.eu/environment/waste/studies/pdf/heavy_metalsreport.pdf | ||
| + | |||
| + | 2.Duruibe, J. O., Ogwuegbu, M. O. C. and Egwurugwu, J. N. 2007. Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences. | ||
| + | |||
| + | 3.World Health Organization. 2011. Guideline for drinking-water quality. URL: http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf | ||
| + | |||
| + | 4. Itai-Itai Disease: A Puzzling Example of Metal Toxicity. URL: http://faculty.virginia.edu/metals/cases/rasnake1.html | ||
| + | |||
| + | 5. The New York times. Rice Tainted With Cadmium Is Discovered in Southern China. URL: http://www.nytimes.com/2013/05/22/world/asia/cadmium-tainted-rice-discovered-in-southern-china.html?_r=0 | ||
</div> | </div> | ||
{{Team:Edinburgh/Footer}} | {{Team:Edinburgh/Footer}} | ||
Latest revision as of 03:47, 5 October 2013
Contents |
Metal toxicity
Biotoxicity
Heavy metal cause poisoning effect on human body by interacting with the normal biochemistry in metabolic process. They are converted to their stable oxidation state (ion) in the stomach after ingested and combine with proteins and enzymes etc. to form strong chemical bonds. The function of the enzyme is therefore inhibited by the poisoning metal, whereas the protein-metal complex reacts with a metabolic enzyme as a substrate. The reaction scheme below indicates that enzyme (E) and substrate(S) react in either the lock-and-key or the induced fit pattern. In either case, a substrate combines with an enzyme in a highly specific way to form an activated enzyme-substrate complex (ES*).
![]()
An enzyme cannot interact with other substrates until it is freed (state E). In multi-enzyme complex (consisting of 3 or more enzymes) which includes enzymes for an entire sequence, reaction between the product from one enzyme and a second one takes place in a chain process. The final enzyme yields the final product as indicated in the equation below.
![]()
The final produce F inhibits further actions by reacting with E1 since it does not exist at the beginning of the reaction. Hence, E1 is not able to interact with any other substrate until F is utilized by the human body. If the body is not capable of utilizing the heavy metal –protein substrate, the related enzyme will be permanently blocked (F and E1 respectively), which then is not able to initiate any bio-reaction or function. Therefore, heavy metal remains in the tissue and results in bio-dysfunctions of various gravities. Moreover, a metal ion can easily replace the metal ion in the body’s metallo-enzyme, if they have the similar size. For example, Zn2+ in some dehydrogenating enzyme can be conveniently replaced by Cd 2+, leading to Cadmium toxicity. The most toxic form of metals in the most stable oxidation states (Cd 2+ and Pb 2+ etc.). In the most stable oxidation states, they can form very stable biotoxic compound, which is very difficult to be dissociated from metal-biomolecule compound.
Human exposure
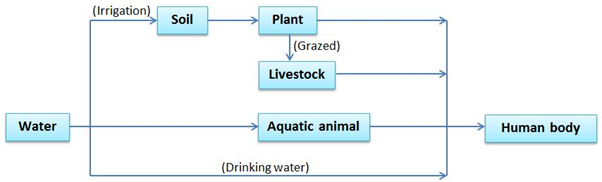
Humans are directly exposed to heavy metal by inhalation of metal dust and gas (Pb), as well as intake of metal-contaminated water (Cd and Hg). However, protection from metal dust and contaminated water is not sufficient to avoid exposures to heavy metals.
Industries including mining, papermaking, leather and electroplating etc., release contaminated water to the environment. Soils (including agricultural soil) are therefore polluted by rain or irrigation water where heavy metals are dissolved. The metals are taken up by plants and accumulate in their tissues. Heavy metals will also accumulate in bodies of animals if they are grazed by contaminated plant or raised in water body that are contaminated by metal ions. Humans are therefore indirectly exposed to heavy metals by consuming contaminated plants or animal. In summary, all living organisms within a given ecosystem are contaminated to some extent according to their position in the food chain.
Metal poisoning
Cadmium
According to the final report Heavy Metal in Waste published by Europe Commission in 2002, Cadmium accumulates in human body (especially in kidney) and leads to dysfunction of the kidney with impaired reabsorption of, for example, proteins, glucose and amino acids. Furthermore, skeletal damages (e.g. osteomalacia and osteoporosis etc.) are believed to be a critical effect of cadmium exposure indicated by studies on both humans and animal.
Other effects of cadmium exposure are disturbances in calcium metabolism, hypercalciuria and formation of renal stones.
The most notorious cadmium poisoning case is Itai-itai disease, starting around 1912 in Japan. It was caused by the considerable amount of Cadmium released in the rivers (Jinzu River) by mining companies and a mass population was seriously affected by the disease. People were exposed to cadmium through drinking water, eating contaminated rice and vegetables grown on polluted soil. Those who were affected suffered from osteomalacia (softening of the bones) and osteoporosis (loss of bone mass and weakness). Fractures were more common as the bone weakens. In extremely serious cases, Cadmium poisoning led to death after causing a wide range of damage on human body. Also earlier this year (June, 2013), it was reported that rice tainted with Cadmium is discovered in southern China, leading to swell in China rice import. The news indicates that heavy metal contamination (in water and soil) is still an issue in many countries, especially developing countries such India and China, where economic development outweighs environmental protection.
Clinical effect of other metal poisoning on humans
| Cr | 1.Irritating respiratory effects, possible circulatory effects, effects on stomach and blood , liver and kidney effects, and increased risk of death from lung cancer
2. Allergic responses (e.g., asthma and dermatitis) in sensitised individuals |
| Fe | 1. aggravates the risks of diabetes, liver cancer, heart disease and arthritis
2. enlarged liver, skin pigmentation, joint diseases, loss of body hair, amenorrhea and impotence |
| Mn | 1. Long-term exposure to excessive level leads to iron-deficiency anemia
2. Increased manganese intake impairs the activity of copper metallo-enzymes 3. Parkinson like symptoms 4. Hypertension in patients more than 40 |
| Pb | 1. Biological effects depending on the level and duration of exposure. Effects may range from inhibition of enzymes to the production of marked morphological changes and death
2. Effect on the central nervous system. Epidemiological studies suggest that low level exposure of the foetus and developing child may lead to reprotoxic effects, i.e. damage to the learning capacity and the neuropsychological development 3. Effects on haemoglobin synthesis and anaemia observed in children at lead blood levels above 40 µg/dl 4. Kidney damages |
| Zn | 1. Similar sign of illness as Cr
2. Cause system dysfunctions that result in impairment of growth and reproduction |
Drinking water quality guidelines
Access to safe drinking-water is important as a health and development issue at a national, regional and local level. WHO has published a guideline, which includes the provisional maximum concentration of metal ion in drinking water. The following table demonstrates the guideline value for different heavy metal.
| Element | Guideline value |
| Cd | 0.003mg/litre |
| Cr | 0.05mg/litre |
| Fe | 0.3mg/litre |
| Mn | 0.4mg/litre |
| Ni | 0.07mg/litre |
| Pb | 0.01mg/litre |
References
1.European Commission. 2002. Heavy metal in waste. URL: http://ec.europa.eu/environment/waste/studies/pdf/heavy_metalsreport.pdf
2.Duruibe, J. O., Ogwuegbu, M. O. C. and Egwurugwu, J. N. 2007. Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences.
3.World Health Organization. 2011. Guideline for drinking-water quality. URL: http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf
4. Itai-Itai Disease: A Puzzling Example of Metal Toxicity. URL: http://faculty.virginia.edu/metals/cases/rasnake1.html
5. The New York times. Rice Tainted With Cadmium Is Discovered in Southern China. URL: http://www.nytimes.com/2013/05/22/world/asia/cadmium-tainted-rice-discovered-in-southern-china.html?_r=0

| 
| | | | 
|
| This iGEM team has been funded by the MSD Scottish Life Sciences Fund. The opinions expressed by this iGEM team are those of the team members and do not necessarily represent those of MSD | |||||
 "
"