Team:Newcastle/Project
From 2013.igem.org
| (139 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | {{Team:Newcastle}} | |
| - | =Project= | + | =Project Overview= |
| - | + | [[File:SyntheticBiologyEngineeringLifeCycle.jpg|500px|center]] | |
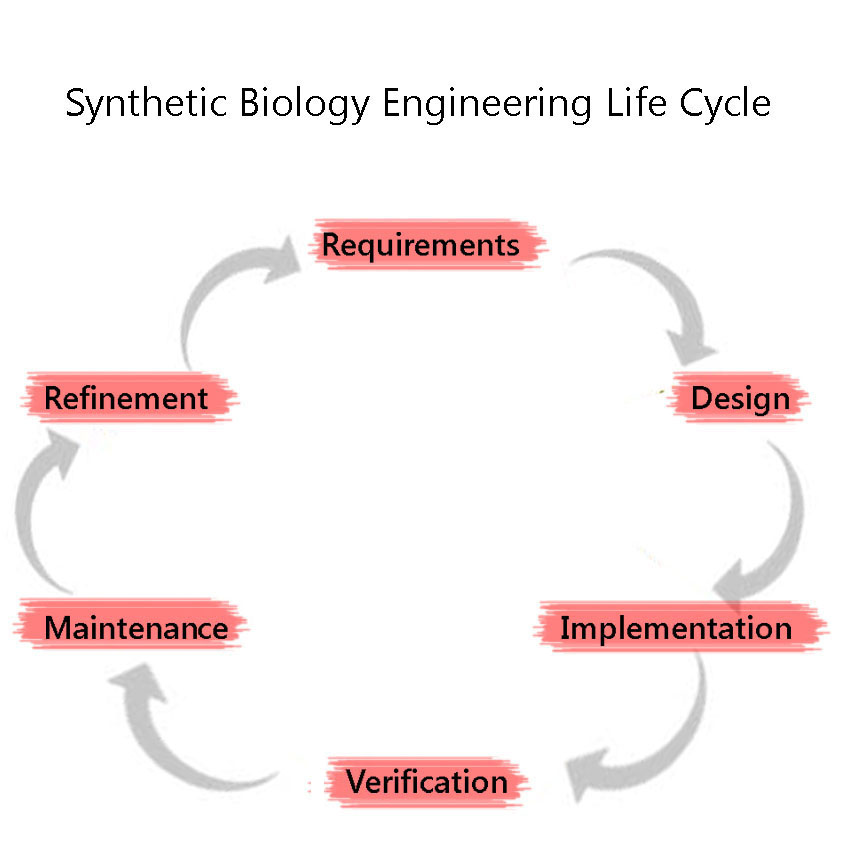
| - | + | Synthetic Biology is a discipline which heavily employs engineering principles. One of these principles is the Engineering Lifecycle, a framework in which the project is split into clearly defined sections, based on the development of the project. These are, in sequential order: | |
| + | #Requirements | ||
| + | #Design (including Modelling) | ||
| + | #Implementation | ||
| + | #Verification | ||
| + | #Maintenance | ||
| + | #Refinement | ||
| + | The cycle is iterated through again, as we attempt to improve the system further. We adhered to this cycle throughout our project including the development and characterisation of our BioBricks. This included modelling our BioBricks and sub-project outcomes before any experiments were conducted. This helped us better understand our engineered systems and predict the results of our ‘wet’ lab experiments. | ||
| - | + | Click on the links below to view each model or visit our [https://2013.igem.org/Team:Newcastle/Modelling/Introduction introductory modelling page]: | |
| + | *[https://2013.igem.org/Team:Newcastle/Modelling/L-form_Switch L-form switch] | ||
| + | *[https://2013.igem.org/Team:Newcastle/Modelling/CellShapeModel Cell Shape] | ||
| + | *[https://2013.igem.org/Team:Newcastle/Modelling/Cell_Fusion Cell Fusion] | ||
| + | *[https://2013.igem.org/Team:Newcastle/Modelling/Hbsu_Fusion_Protein Hbsu-xFP] | ||
| - | == | + | ==A Foundational Advance== |
| - | + | [[File:BareCillus_Switch.png|700px]] | |
| - | + | While researching Synthetic Biology we found that a cell wall, one of the standard components of the bacterial cell, often causes difficulties in many techniques. These include transformation efficiency, secretion of recombinant proteins, adaption to the environment etc. So we thought to ourselves: is there any way to remove the cell wall and still have a viable cell? | |
| - | + | As a result we have developed a new chassis with the potential to revolutionize how Synthetic Biology is performed. The main [https://2013.igem.org/Team:Newcastle/Parts/l_form_switch BioBrick(BBa_K1185000)] that we have introduced enables the switching on and off of the bacterial cell wall in the model gram positive bacteria ''Bacillus subtilis'', at the demand of the synthetic biologist, while still allowing cells to grow and divide. Employing bacterial cells without a cell wall can both enable the synthetic biologist to explore new applications and research areas, and also build-upon and improve areas that are already being explored in Synthetic Biology. Rather than the application-oriented nature of many iGEM projects, we think that the use of cell wall-less bacteria as a novel chassis in Synthetic Biology, as we propose, can benefit across the whole subject area, and furthermore be utilised as a tool to allow for even greater feats to be achieved by future iGEM teams. | |
| - | + | Bacteria which have lost their cell wall yet are still able to grow and divide are called L-forms, or as we prefer to call them, naked bacteria. If you would like to learn more about L-forms, please take a look at our [https://2013.igem.org/Team:Newcastle/Project/L_forms L-form page] or click [http://www.youtube.com/watch?v=b0Kk6bKKOQ0 here] to watch a short video summarising our project. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Once we had created L-forms with our [https://2013.igem.org/Team:Newcastle/Parts/l_form_switch Switch BioBrick], we were unable to ignore the new opportunities that L-forms bring to Synthetic Biology: | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ====[https://2013.igem.org/Team:Newcastle/Project/shuffling_endosymbiosis Genome Shuffling]==== | |
| - | + | ||
| - | + | We investigated using genome shuffling and L-forms to artificially evolve ''B. subtilis''. Genome shuffling increases the rate of evolution, allowing the improvement of any biological system or phenotype in a feasible time frame. Over the past eight years iGEM teams have dreamt up innovative ways of harnessing Synthetic Biology. However this relatively new field faces challenges such as producing high quality yield of the desired product. Using L-forms allows the use of genome shuffling to solve this problem. If harnessed this could improve the efficiency of hundreds of iGEM projects as well as cell factories across the field of synthetic biology. | |
| - | |||
| - | + | [[File:BareCillus_Genome_shuffling.png|700px]] | |
| - | |||
| - | |||
| - | + | ====[https://2013.igem.org/Team:Newcastle/Project/plants Introduction and Detection of Naked Bacteria in Plants]==== | |
| - | + | We successfully inoculated HBsu-GFP tagged L-forms into ''Brassica pekinens'' (Chinese Cabbage). L-forms have been shown to form symbiotic relationships in plants such as Chinese Cabbage and strawberries. Plants with naked bacteria show [http://www.ncbi.nlm.nih.gov/pubmed/11849491 increased resistance to fungus] and L-forms could be engineered to deliver useful compounds to crops; an artificial symbiotic relationship. In the future L-forms could be engineered to provide their host plants with beneficial compounds such as nitrogen, plant hormones or anti-fungals. This could give better crop yields, more nutritious harvests and reduce the need for spraying of fertilizer or pesticides. Crucially, we have shown that L-forms will burst if they are not in an osmotically stable environment. This makes them a better delivery system than cell walled bacteria as they will die if they exit their host plant. | |
| - | + | ||
| - | |||
| - | |||
| - | + | [[File:BareCillus_Plant_infographic.png|700px]] | |
| - | + | ||
| - | + | ====[https://2013.igem.org/Team:Newcastle/Project/shape_shifting Shape Shifting]==== | |
| - | + | The loss of the cell wall leaves L-forms protected by only a fluid cell membrane. The advantage of this is that these cells would be able to adapt to shapes of various cracks and cavities, or will be able to "squeeze through" tiny channels and deliver cargo to hard-to reach targets. | |
| - | + | To explore the potential for L-forms in this area, we intended to manipulate them using microfluidics. For this we created microfluidics chips using autoCAD. We have laid the foundations for future iGEM teams to further explore the biophysical properties of bacterial cell membranes. | |
| - | + | ||
| - | |||
| - | + | ||
| + | [[File:Shape shifting copy.jpg|700px]] | ||
| + | |||
| + | |||
| + | This isn’t a finite list of what can be done with naked bacteria, there’s loads more! L-forms are currently used to discover novel antibiotics which don’t act on the cell wall. L-forms can also teach us a great deal about how bacterial life has evolved, through acting as a model for a cell wall-less bacterial progenitor, and allowing testing of induction of endosymbiosis in cell wall-less organisms [https://2013.igem.org/Team:Newcastle/Project#References (Mercier et al. 2013)]. | ||
| + | |||
| + | |||
| + | In addition to our work above, we characterized [http://parts.igem.org/Part:BBa_K818000:Experience Part: BBa_K818000] showing that it does not work in ''B. subtilis''. We then sequenced this part, noted that its sequence differed from its entry on the iGEM registry and created a new registry page for [http://parts.igem.org/Part:BBa_K1185004 Part: BBa_K1185004] with the correct sequence. | ||
| + | |||
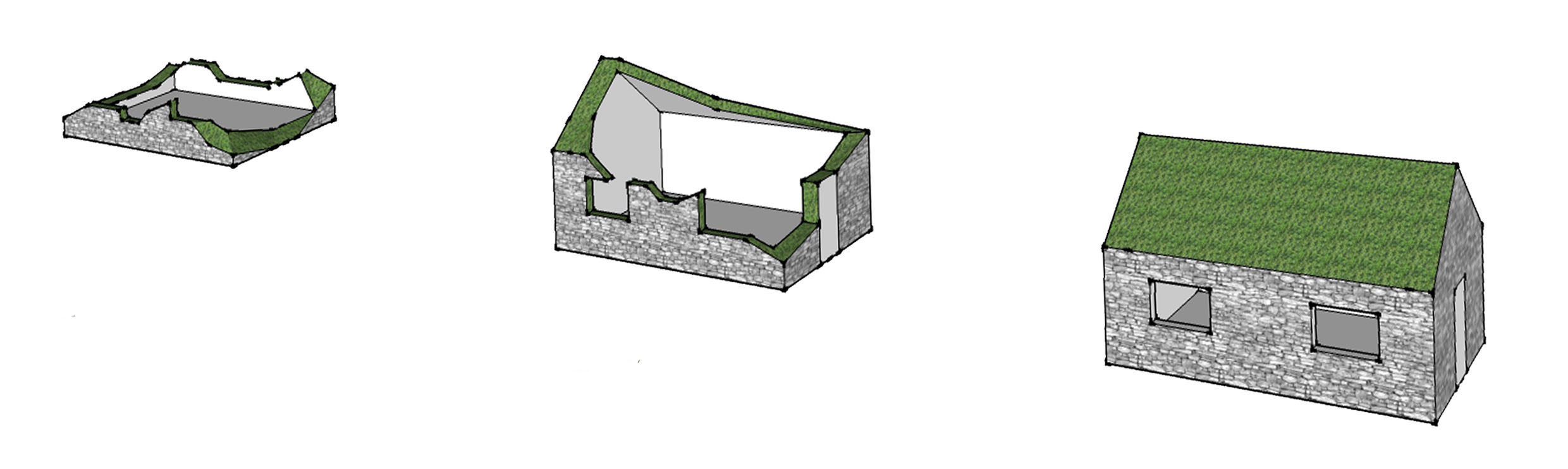
| + | ====[https://2013.igem.org/Team:Newcastle/Architecture Architecture]==== | ||
| + | |||
| + | Because Synthetic Biology applies engineering techniques to biology, it makes it’s design cycle very similar to architecture and modelling is a very important stage in the design cycle of both fields. Just like we used models in our project to predict outcomes, computer aided design modelling is used in architecture. This got us thinking about other similarities and differences in the design cycle. We then speculated on the possible ways we could apply Synthetic Biology to architecture and the effect the two fields may have on each other in the future. | ||
| + | |||
| + | |||
| + | [[File:Arch_copy.jpg|600px]] | ||
==References== | ==References== | ||
| - | |||
| - | Chang S and Cohen SN (1979)High frequency transformation of ''Bacillus subtilis'' protoplasts by plasmid DNA. ''Molecular Genetics & Genomics'', '''168''', 111–115. | + | [http://www.ncbi.nlm.nih.gov/pubmed/11849491 Walker R, Ferguson CMJ, Booth NA and Allan EJ (2002) The symbiosis of ''Bacillus subtilis'' L-forms with Chinese cabbage seedlings inhibits conidial germination of ‘''Botrytis cinerea''. ''Letters in Applied Microbiology'', '''34''', 42-45.] |
| + | |||
| + | [http://www.ncbi.nlm.nih.gov/pubmed/107388 Chang S and Cohen SN (1979)High frequency transformation of ''Bacillus subtilis'' protoplasts by plasmid DNA. ''Molecular Genetics & Genomics'', '''168''', 111–115.] | ||
| + | |||
| + | [http://www.cell.com/abstract/S0092-8674(13)00135-9 Mercier R, Kawai Y and Errington J. (2013) Excess Membrane Synthesis Drives a Primitive Mode of Cell Proliferation, ''Cell'', '''152''', 997–1007.] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{{Team:Newcastle/Sponsors}} | {{Team:Newcastle/Sponsors}} | ||
Latest revision as of 17:57, 28 October 2013

Contents |
Project Overview
Synthetic Biology is a discipline which heavily employs engineering principles. One of these principles is the Engineering Lifecycle, a framework in which the project is split into clearly defined sections, based on the development of the project. These are, in sequential order:
- Requirements
- Design (including Modelling)
- Implementation
- Verification
- Maintenance
- Refinement
The cycle is iterated through again, as we attempt to improve the system further. We adhered to this cycle throughout our project including the development and characterisation of our BioBricks. This included modelling our BioBricks and sub-project outcomes before any experiments were conducted. This helped us better understand our engineered systems and predict the results of our ‘wet’ lab experiments.
Click on the links below to view each model or visit our introductory modelling page:
A Foundational Advance
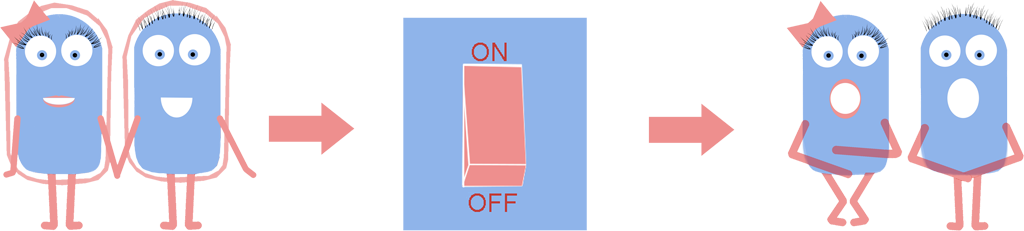
While researching Synthetic Biology we found that a cell wall, one of the standard components of the bacterial cell, often causes difficulties in many techniques. These include transformation efficiency, secretion of recombinant proteins, adaption to the environment etc. So we thought to ourselves: is there any way to remove the cell wall and still have a viable cell?
As a result we have developed a new chassis with the potential to revolutionize how Synthetic Biology is performed. The main BioBrick(BBa_K1185000) that we have introduced enables the switching on and off of the bacterial cell wall in the model gram positive bacteria Bacillus subtilis, at the demand of the synthetic biologist, while still allowing cells to grow and divide. Employing bacterial cells without a cell wall can both enable the synthetic biologist to explore new applications and research areas, and also build-upon and improve areas that are already being explored in Synthetic Biology. Rather than the application-oriented nature of many iGEM projects, we think that the use of cell wall-less bacteria as a novel chassis in Synthetic Biology, as we propose, can benefit across the whole subject area, and furthermore be utilised as a tool to allow for even greater feats to be achieved by future iGEM teams.
Bacteria which have lost their cell wall yet are still able to grow and divide are called L-forms, or as we prefer to call them, naked bacteria. If you would like to learn more about L-forms, please take a look at our L-form page or click [http://www.youtube.com/watch?v=b0Kk6bKKOQ0 here] to watch a short video summarising our project.
Once we had created L-forms with our Switch BioBrick, we were unable to ignore the new opportunities that L-forms bring to Synthetic Biology:
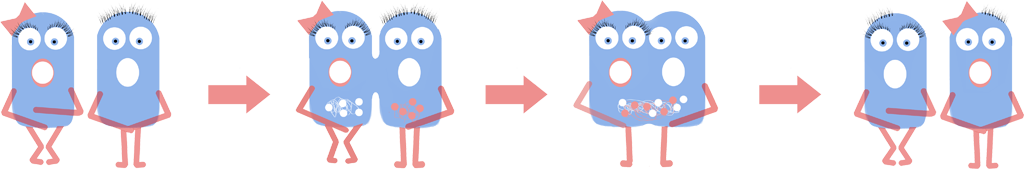
Genome Shuffling
We investigated using genome shuffling and L-forms to artificially evolve B. subtilis. Genome shuffling increases the rate of evolution, allowing the improvement of any biological system or phenotype in a feasible time frame. Over the past eight years iGEM teams have dreamt up innovative ways of harnessing Synthetic Biology. However this relatively new field faces challenges such as producing high quality yield of the desired product. Using L-forms allows the use of genome shuffling to solve this problem. If harnessed this could improve the efficiency of hundreds of iGEM projects as well as cell factories across the field of synthetic biology.
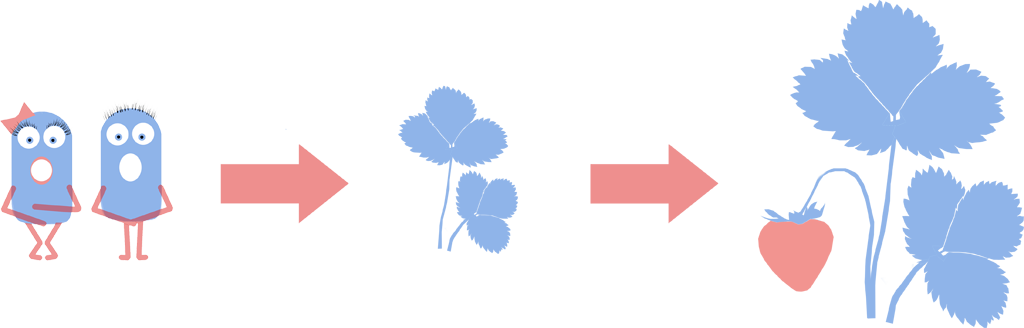
Introduction and Detection of Naked Bacteria in Plants
We successfully inoculated HBsu-GFP tagged L-forms into Brassica pekinens (Chinese Cabbage). L-forms have been shown to form symbiotic relationships in plants such as Chinese Cabbage and strawberries. Plants with naked bacteria show [http://www.ncbi.nlm.nih.gov/pubmed/11849491 increased resistance to fungus] and L-forms could be engineered to deliver useful compounds to crops; an artificial symbiotic relationship. In the future L-forms could be engineered to provide their host plants with beneficial compounds such as nitrogen, plant hormones or anti-fungals. This could give better crop yields, more nutritious harvests and reduce the need for spraying of fertilizer or pesticides. Crucially, we have shown that L-forms will burst if they are not in an osmotically stable environment. This makes them a better delivery system than cell walled bacteria as they will die if they exit their host plant.
Shape Shifting
The loss of the cell wall leaves L-forms protected by only a fluid cell membrane. The advantage of this is that these cells would be able to adapt to shapes of various cracks and cavities, or will be able to "squeeze through" tiny channels and deliver cargo to hard-to reach targets. To explore the potential for L-forms in this area, we intended to manipulate them using microfluidics. For this we created microfluidics chips using autoCAD. We have laid the foundations for future iGEM teams to further explore the biophysical properties of bacterial cell membranes.
This isn’t a finite list of what can be done with naked bacteria, there’s loads more! L-forms are currently used to discover novel antibiotics which don’t act on the cell wall. L-forms can also teach us a great deal about how bacterial life has evolved, through acting as a model for a cell wall-less bacterial progenitor, and allowing testing of induction of endosymbiosis in cell wall-less organisms (Mercier et al. 2013).
In addition to our work above, we characterized [http://parts.igem.org/Part:BBa_K818000:Experience Part: BBa_K818000] showing that it does not work in B. subtilis. We then sequenced this part, noted that its sequence differed from its entry on the iGEM registry and created a new registry page for [http://parts.igem.org/Part:BBa_K1185004 Part: BBa_K1185004] with the correct sequence.
Architecture
Because Synthetic Biology applies engineering techniques to biology, it makes it’s design cycle very similar to architecture and modelling is a very important stage in the design cycle of both fields. Just like we used models in our project to predict outcomes, computer aided design modelling is used in architecture. This got us thinking about other similarities and differences in the design cycle. We then speculated on the possible ways we could apply Synthetic Biology to architecture and the effect the two fields may have on each other in the future.
References
[http://www.ncbi.nlm.nih.gov/pubmed/11849491 Walker R, Ferguson CMJ, Booth NA and Allan EJ (2002) The symbiosis of Bacillus subtilis L-forms with Chinese cabbage seedlings inhibits conidial germination of ‘Botrytis cinerea. Letters in Applied Microbiology, 34, 42-45.]
[http://www.ncbi.nlm.nih.gov/pubmed/107388 Chang S and Cohen SN (1979)High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Molecular Genetics & Genomics, 168, 111–115.]
[http://www.cell.com/abstract/S0092-8674(13)00135-9 Mercier R, Kawai Y and Errington J. (2013) Excess Membrane Synthesis Drives a Primitive Mode of Cell Proliferation, Cell, 152, 997–1007.]
 "
"