Team:UGent/LiteratureStudy
From 2013.igem.org
| (4 intermediate revisions not shown) | |||
| Line 23: | Line 23: | ||
</td> | </td> | ||
<td> | <td> | ||
| - | <p></html>[[File:UGent_2013_AlleleSegregation.png|250px| | + | <p></html>[[File:UGent_2013_AlleleSegregation.png|thumb|250px|Allele segregation results in a rapid loss of productivity in plasmids]]<html> |
| - | + | <br></p> | |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 40: | Line 40: | ||
{{:Team:UGent/Templates/ToggleBoxStart}} Read more about CIChE{{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | {{:Team:UGent/Templates/ToggleBoxStart}} Read more about CIChE{{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | ||
<html> | <html> | ||
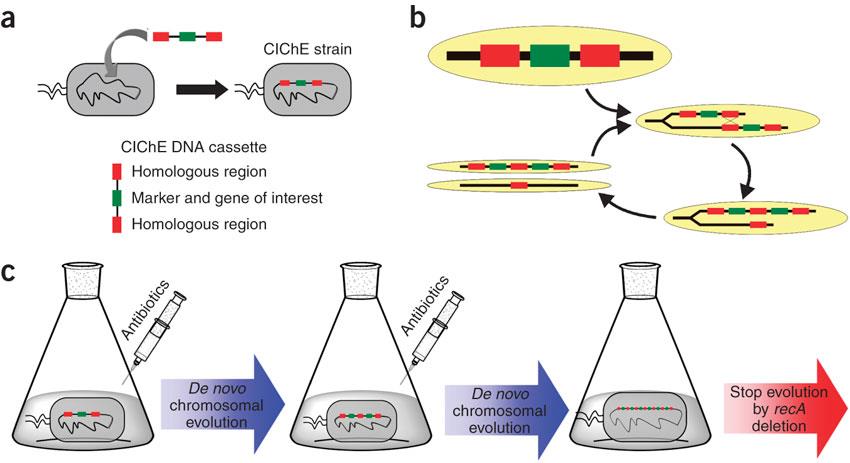
| - | <p>In 2009, Tyo <i>et al.</i> developed a technique for the stable, high copy expression of a gene of interest in E. coli without the use of high copy number plasmids, thus avoiding their previously stated negative characteristics. They called this plasmid-free, high gene copy expression system ‘chemically inducible chromosomal evolution’. In this method, the gene of interest is integrated in the microbial genome and then amplified to achieve multiple copies and reach the desired expression level. Genomic integration guarantees ordered inheritance, resolving the problem of allele segregation.</p> | + | <p>In 2009, Tyo <i>et al.</i> developed a technique for the stable, high copy expression of a gene of interest in <i>E. coli</i> without the use of high copy number plasmids, thus avoiding their previously stated negative characteristics. They called this plasmid-free, high gene copy expression system ‘chemically inducible chromosomal evolution’. In this method, the gene of interest is integrated in the microbial genome and then amplified to achieve multiple copies and reach the desired expression level. Genomic integration guarantees ordered inheritance, resolving the problem of allele segregation.</p> |
<p>CIChE works as follows: First a construct, containing the gene(s) of interest and the antibiotic marker <i>chloramphenicol acetyl transferase</i> (<i>cat</i>) flanked by homologous regions, is delivered to and subsequently integrated into the <i>E. coli</i> genome. The construct can be amplified in the genome through tandem gene duplication by <i>recA</i> homologous recombination. Then the strain is cultured in increasing concentrations of chloramphenicol, providing a growth advantage for cells with increased repeats of the construct and thereby selecting for bacteria with a higher gene copy number (<b>Figure</b>).</p> | <p>CIChE works as follows: First a construct, containing the gene(s) of interest and the antibiotic marker <i>chloramphenicol acetyl transferase</i> (<i>cat</i>) flanked by homologous regions, is delivered to and subsequently integrated into the <i>E. coli</i> genome. The construct can be amplified in the genome through tandem gene duplication by <i>recA</i> homologous recombination. Then the strain is cultured in increasing concentrations of chloramphenicol, providing a growth advantage for cells with increased repeats of the construct and thereby selecting for bacteria with a higher gene copy number (<b>Figure</b>).</p> | ||
</html> | </html> | ||
| Line 124: | Line 124: | ||
<td> | <td> | ||
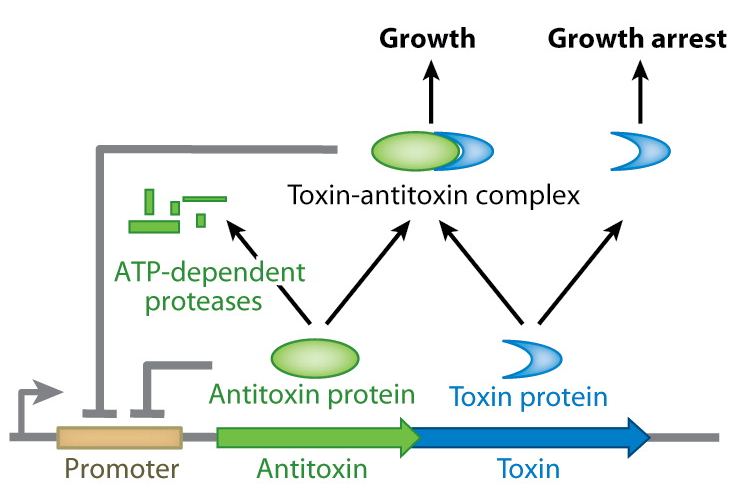
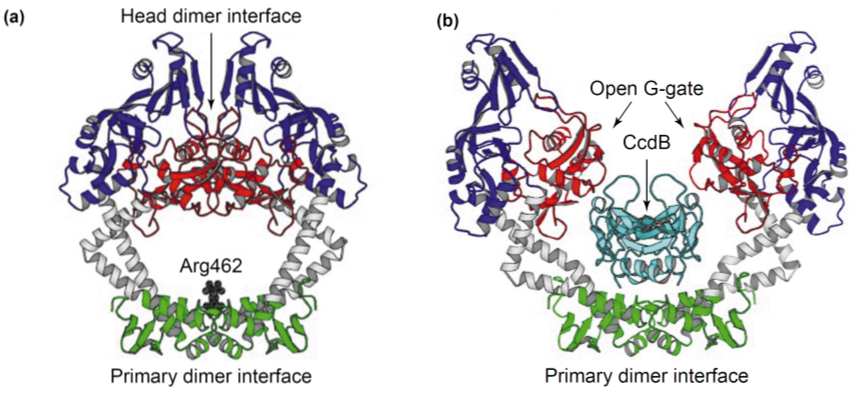
<p>In the absence of its antidote CcdA, CcdB causes reduced DNA synthesis, activation of the SOS pathway, cell filamentation and eventually cell death. When CcdB interacts with a gyrase:DNA complex, it stabilises the cleavable complex. In this mode of action, CcdB acts as a poison, promoting DNA breakage mediated by gyrase. The antidote CcdA can inhibit CcdB by the formation of a tight non-covalent complex. This prevents CcdB from making a covalent complex with gyrase. But even after CcdB has formed a complex with gyrase, CcdA can still reverse the blocking CcdB caused.</p> | <p>In the absence of its antidote CcdA, CcdB causes reduced DNA synthesis, activation of the SOS pathway, cell filamentation and eventually cell death. When CcdB interacts with a gyrase:DNA complex, it stabilises the cleavable complex. In this mode of action, CcdB acts as a poison, promoting DNA breakage mediated by gyrase. The antidote CcdA can inhibit CcdB by the formation of a tight non-covalent complex. This prevents CcdB from making a covalent complex with gyrase. But even after CcdB has formed a complex with gyrase, CcdA can still reverse the blocking CcdB caused.</p> | ||
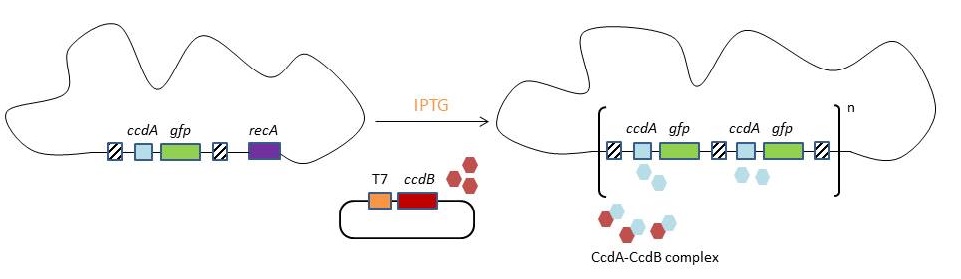
| - | <p>As CcdA has no toxic effects in the absence of CcdB, the ccdA gene will be used as selectable marker integrated in the CIChE construct, under the control of a constitutive promoter. By adding a plasmid bearing the ccdB gene under the control of a chemically inducible promoter, we suggest it is possible to regulate the amount of CcdB present in the cell titrating the chemical inducer. If this CIChE system works, cells with a higher gene copy number could be attained by titrating higher concentrations of the chemical inducer, as these have more CcdA to compensate for the toxic CcdB.</p> | + | <p>As CcdA has no toxic effects in the absence of CcdB, the <i>ccdA</i> gene will be used as selectable marker integrated in the CIChE construct, under the control of a constitutive promoter. By adding a plasmid bearing the ccdB gene under the control of a chemically inducible promoter, we suggest it is possible to regulate the amount of CcdB present in the cell titrating the chemical inducer. If this CIChE system works, cells with a higher gene copy number could be attained by titrating higher concentrations of the chemical inducer, as these have more CcdA to compensate for the toxic CcdB.</p> |
</td> | </td> | ||
<td> | <td> | ||
| Line 140: | Line 140: | ||
<h2>Construct</h2> | <h2>Construct</h2> | ||
| - | <p> A construct containing the homologous regions, the antitoxin <i>ccdA</i> and <i>gfp</i> will be inserted in the genome of an <i>E. coli</i> strain which contains <i>recA</i>. <i>recA</i> is necessary to perform the tandem gene duplication by homologous recombination. When the desired copy number is reached, <i>recA</i> is deleted. A plasmid containing the <i>ccdB</i> toxin gene is transformed into the cell. Expression of <i>ccdB</i> is controlled by the T7-promoter. The <b>T7 promoter</b> is not recognised by the <i>E. coli</i> RNA polymerase. By using an inducible promoter to regulate the production of T7 RNAP, it is possible to change the amount of transcription of the ccdB toxin.By raising IPTG (inducer) concentrations, the selection pressure increases and chromosomal evolution is achieved.</p> | + | <p> A construct containing the homologous regions, the antitoxin <i>ccdA</i> and <i>gfp</i> will be inserted in the genome of an <i>E. coli</i> strain which contains <i>recA</i>. <i>recA</i> is necessary to perform the tandem gene duplication by homologous recombination. When the desired copy number is reached, <i>recA</i> is deleted. A plasmid containing the <i>ccdB</i> toxin gene is transformed into the cell. Expression of <i>ccdB</i> is controlled by the T7-promoter. The <b>T7 promoter</b> is not recognised by the <i>E. coli</i> RNA polymerase. By using an inducible promoter to regulate the production of T7 RNAP, it is possible to change the amount of transcription of the <i>ccdB</i> toxin. By raising IPTG (inducer) concentrations, the selection pressure increases and chromosomal evolution is achieved.</p> |
</html> | </html> | ||
Latest revision as of 01:45, 5 October 2013
|
You can read our entire literature study about our new model for stabilised gene duplication here. Below you can read the most important aspects of it. Introduction: high gene expression in industrial biotechnologyThe main goal of industrial biotechnology is to increase the yield of biochemical products using microorganisms as production hosts. This includes engineering large synthetic pathways and improving their expression. Overexpression of genes has mainly been achieved by using high or medium copy plasmids. However, studies have demonstrated that plasmid-bearing cells lose their productivity fairly quickly as a result of genetic instability.
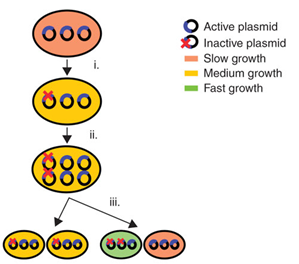
In industrial biotechnology, a common technique to express new synthetic products and pathways is the use of plasmids as vectors. Plasmids are circular pieces of DNA. They are easy to insert into cells and replicate independently from the genome, allowing strong gene expression. Overexpression is easily achieved by using plasmids with a medium or high copy number, different promoter systems, ribosome binding sites (RBS), etc. Thanks to plasmids, the industrial biotechnology has grown substantially over the past years. However, the use of plasmids entails some important disadvantages: plasmid maintenance imposes a metabolic burden on cells and plasmids suffer from genetic instability. Metabolic burdenWhen plasmids are present in cells and replicate, they create a metabolic burden. This is defined by Bentley et al. (1990) as “the amount of resources that are taken from the host cell metabolism for foreign DNA maintenance and replication”. As a result, metabolic load causes many alterations to the physiology and metabolism of the cell and reduces the cellular fitness. The most common change is a delayed growth. This can be caused by the fact that new pathways for energy generation are activated due to the competition between cell propagation and plasmid replication. This growth retardation leads to a lower yield of the desired product. Genetic instabilityPlasmids are genetically instable due to three processes: segregational instability, structural instability and allele segregation.
Therefore a new method was developed for the overexpression of a gene of interest in the bacterial chromosome: Chemically Inducible Chromosomal evolution (CIChE). In this technique the chromosome is evolved to contain a higher number of gene copies by adding a chemical inducer.
In 2009, Tyo et al. developed a technique for the stable, high copy expression of a gene of interest in E. coli without the use of high copy number plasmids, thus avoiding their previously stated negative characteristics. They called this plasmid-free, high gene copy expression system ‘chemically inducible chromosomal evolution’. In this method, the gene of interest is integrated in the microbial genome and then amplified to achieve multiple copies and reach the desired expression level. Genomic integration guarantees ordered inheritance, resolving the problem of allele segregation. CIChE works as follows: First a construct, containing the gene(s) of interest and the antibiotic marker chloramphenicol acetyl transferase (cat) flanked by homologous regions, is delivered to and subsequently integrated into the E. coli genome. The construct can be amplified in the genome through tandem gene duplication by recA homologous recombination. Then the strain is cultured in increasing concentrations of chloramphenicol, providing a growth advantage for cells with increased repeats of the construct and thereby selecting for bacteria with a higher gene copy number (Figure).
This process is called chromosomal evolution. When the desired gene copy number is reached recA is deleted, thereby fixing the copy number. The use of CIChE-strains poses several advantages. They require no selection markers and can be cultured without the addition of antibiotics to the medium. This is in contrast to strains containing plasmids, which still need antibiotic selection. Also, in CIChE-strains yields can be tuned by varying the antibiotic concentration during chromosomal evolution. CloseIt has been shown that approximately 40 and even up to 50 gene copies can be attained using chromosomal evolution. It has also been demonstrated that, while plasmid-bearing strains lose their productivity after 40 generations due to allele segregation, gene copy number and productivity of CIChE-strains remain stable even after 70 generations. This genetic stability is considered to be the most important asset of CIChE. The original model for CIChE, however, results in bacterial strains containing a large number of antibiotic resistance genes. To make this valuable technique more widely applicable in the industry, we developed a model for chromosomal evolution based on a toxin-antitoxin system instead of antibiotic resistance. | |||
 "
"