Team:Yale/Project Validate
From 2013.igem.org
(→Positive Selection with KanR) |
|||
| (23 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
<div id="header">{{Template:Team:Yale2013/Templates/Header}}</div> | <div id="header">{{Template:Team:Yale2013/Templates/Header}}</div> | ||
| - | + | <!-- Project Submenu adapted from cssmenumaker --> | |
| - | + | <html> | |
| - | + | <style type = "text/css"> | |
| - | + | #cssmenu1 ul, | |
| - | + | #cssmenu1 li, | |
| - | + | #cssmenu1 span, | |
| - | + | #cssmenu1 a { | |
| - | + | margin: 0; | |
| - | + | padding: 0; | |
| - | + | position: relative; | |
| - | = | + | } |
| - | + | #cssmenu1 { | |
| - | + | height: 39px; | |
| - | + | border-radius: 0px 0px 4px 4px; | |
| - | + | -moz-border-radius: 0px 0px 4px 4px; | |
| - | + | -webkit-border-radius: 0px 0px 4px 4px; | |
| - | < | + | background: #fefefe; |
| - | + | background: -moz-linear-gradient(top, #fefefe 0%, #eee9f0 100%); | |
| + | background: -webkit-gradient(linear, left top, left bottom, color-stop(0%, #fefefe), color-stop(100%, #eee9f0)); | ||
| + | background: -webkit-linear-gradient(top, #fefefe 0%, #eee9f0 100%); | ||
| + | background: -o-linear-gradient(top, #fefefe 0%, #eee9f0 100%); | ||
| + | background: -ms-linear-gradient(top, #fefefe 0%, #eee9f0 100%); | ||
| + | background: linear-gradient(top, #fefefe 0%, #eee9f0 100%); | ||
| + | border-bottom: 2px solid #5763f0; | ||
| + | width: auto; | ||
| + | } | ||
| + | #cssmenu1:after, | ||
| + | #cssmenu1 ul:after { | ||
| + | content: ''; | ||
| + | display: block; | ||
| + | clear: both; | ||
| + | } | ||
| + | #cssmenu1 a { | ||
| + | background: #fefefe; | ||
| + | background: -moz-linear-gradient(top, #fefefe 0%, #ececec 100%); | ||
| + | background: -webkit-gradient(linear, left top, left bottom, color-stop(0%, #fefefe), color-stop(100%, #ececec)); | ||
| + | background: -webkit-linear-gradient(top, #fefefe 0%, #ececec 100%); | ||
| + | background: -o-linear-gradient(top, #fefefe 0%, #ececec 100%); | ||
| + | background: -ms-linear-gradient(top, #fefefe 0%, #ececec 100%); | ||
| + | background: linear-gradient(top, #fefefe 0%, #ececec 100%); | ||
| + | color: #000; | ||
| + | display: inline-block; | ||
| + | font-family: Helvetica, Arial, Verdana, sans-serif; | ||
| + | font-size: 12px; | ||
| + | line-height: 39px; | ||
| + | padding: 0 10px; | ||
| + | text-decoration: none; | ||
| + | } | ||
| + | #cssmenu1 ul { | ||
| + | list-style: none; | ||
| + | } | ||
| + | #cssmenu1 > ul { | ||
| + | float: left; | ||
| + | } | ||
| + | #cssmenu1 > ul > li { | ||
| + | float: left; | ||
| + | } | ||
| + | #cssmenu1 > ul > li > a { | ||
| + | color: #000; | ||
| + | font-size: 12px; | ||
| + | } | ||
| + | #cssmenu1 > ul > li:hover:after { | ||
| + | content: ''; | ||
| + | display: block; | ||
| + | width: 0; | ||
| + | height: 0; | ||
| + | position: absolute; | ||
| + | left: 50%; | ||
| + | bottom: 0; | ||
| + | border-left: 10px solid transparent; | ||
| + | border-right: 10px solid transparent; | ||
| + | border-bottom: 10px solid #5763f0; | ||
| + | margin-left: -10px; | ||
| + | } | ||
| + | #cssmenu1 > ul > li:first-child > a { | ||
| + | border-radius: 0px 0px 4px 4px; | ||
| + | -moz-border-radius: 0px 0px 4px 4px; | ||
| + | -webkit-border-radius: 0px 0px 4px 4px; | ||
| + | } | ||
| + | #cssmenu1 > ul > li.active:after { | ||
| + | content: ''; | ||
| + | display: block; | ||
| + | width: 0; | ||
| + | height: 0; | ||
| + | position: absolute; | ||
| + | left: 50%; | ||
| + | bottom: 0; | ||
| + | border-left: 10px solid transparent; | ||
| + | border-right: 10px solid transparent; | ||
| + | border-bottom: 10px solid #5763f0; | ||
| + | margin-left: -10px; | ||
| + | } | ||
| + | #cssmenu1 > ul > li.active > a { | ||
| + | -moz-box-shadow: inset 0 0 2px rgba(0, 0, 0, 0.1); | ||
| + | -webkit-box-shadow: inset 0 0 2px rgba(0, 0, 0, 0.1); | ||
| + | box-shadow: inset 0 0 2px rgba(0, 0, 0, 0.1); | ||
| + | background: #ececec; | ||
| + | background: -moz-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: -webkit-gradient(linear, left top, left bottom, color-stop(0%, #ececec), color-stop(100%, #ffeeff ef)); | ||
| + | background: -webkit-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: -o-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: -ms-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | } | ||
| + | #cssmenu1 > ul > li:hover > a { | ||
| + | background: #ececec; | ||
| + | background: -moz-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: -webkit-gradient(linear, left top, left bottom, color-stop(0%, #ececec), color-stop(100%, #ffeeff ef)); | ||
| + | background: -webkit-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: -o-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: -ms-linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | background: linear-gradient(top, #ececec 0%, #ffeeff ef 100%); | ||
| + | -moz-box-shadow: inset 0 0 2px rgba(0, 0, 0, 0.1); | ||
| + | -webkit-box-shadow: inset 0 0 2px rgba(0, 0, 0, 0.1); | ||
| + | box-shadow: inset 0 0 2px rgba(0, 0, 0, 0.1); | ||
| + | } | ||
| + | #cssmenu1 .has-sub { | ||
| + | z-index: 1; | ||
| + | } | ||
| + | #cssmenu1 .has-sub:hover > ul { | ||
| + | display: block; | ||
| + | } | ||
| + | #cssmenu1 .has-sub ul { | ||
| + | display: none; | ||
| + | position: absolute; | ||
| + | width: 200px; | ||
| + | top: 100%; | ||
| + | left: 0; | ||
| + | } | ||
| + | #cssmenu1 .has-sub ul li { | ||
| + | *margin-bottom: -1px; | ||
| + | } | ||
| + | #cssmenu1 .has-sub ul li a { | ||
| + | background: #5763f0; | ||
| + | border-bottom: 1px dotted #868ef4; | ||
| + | filter: none; | ||
| + | font-size: 11px; | ||
| + | display: block; | ||
| + | line-height: 120%; | ||
| + | padding: 10px; | ||
| + | color: #ffffff; | ||
| + | } | ||
| + | #cssmenu1 .has-sub ul li:hover a { | ||
| + | background: #2838ec; | ||
| + | } | ||
| + | #cssmenu1 .has-sub .has-sub:hover > ul { | ||
| + | display: block; | ||
| + | } | ||
| + | #cssmenu1 .has-sub .has-sub ul { | ||
| + | display: none; | ||
| + | position: absolute; | ||
| + | left: 100%; | ||
| + | top: 0; | ||
| + | } | ||
| + | #cssmenu1 .has-sub .has-sub ul li a { | ||
| + | background: #2838ec; | ||
| + | border-bottom: 1px dotted #868ef4; | ||
| + | } | ||
| + | #cssmenu1 .has-sub .has-sub ul li a:hover { | ||
| + | background: #1525e6; | ||
| + | } | ||
| + | </style> | ||
| + | <div id="cssmenu1" style="width:830px; margin:0 auto;"> | ||
| + | <ul> | ||
| + | <li><a href='https://2013.igem.org/Team:Yale/Project_Overview'><span>Project Overview</span></a></li> | ||
| + | <li class='active'><a href='https://2013.igem.org/Team:Yale/Project_Validate'><span>Validate PLA Synthesis</span></a></li> | ||
| + | <li><a href='https://2013.igem.org/Team:Yale/Project_Bioassay'><span>Develop Bioassay</span></a></li> | ||
| + | <li><a href='https://2013.igem.org/Team:Yale/Project_MAGE'><span>Apply Mage</span></a></li> | ||
| + | <li><a href='https://2013.igem.org/Team:Yale/Project_Export'><span>Introduce Export System</span></a></li> | ||
| + | <li><a href='https://2013.igem.org/Team:Yale/Project_Bioplastic'><span>Make a Bioplastic</span></a></li> | ||
| + | <li class='last'><a href='https://2013.igem.org/Team:Yale/Project_Collaboration'><span>Collaboration</span></a></li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | </html> | ||
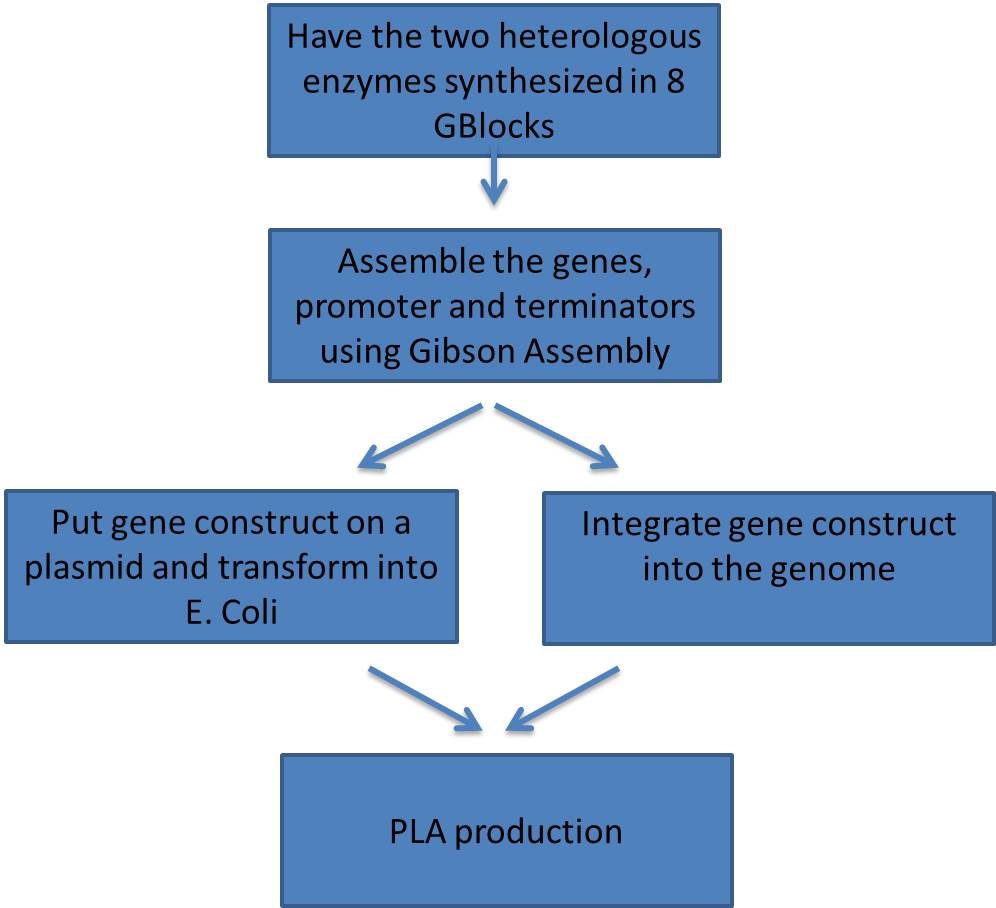
== Engineer strains of ''E. coli'' to validate PLA synthesis == | == Engineer strains of ''E. coli'' to validate PLA synthesis == | ||
| - | |||
===Synthesizing and Assembling the Heterologous Enzymes === | ===Synthesizing and Assembling the Heterologous Enzymes === | ||
*In order to reproduce the results of the Lee group we needed to insert the two heterologous genes. | *In order to reproduce the results of the Lee group we needed to insert the two heterologous genes. | ||
| - | # ''Clostridum propionicum'' propionate CoA transferase (denoted PCT) | + | # ''Clostridum propionicum'' - propionate CoA transferase (denoted PCT) |
| - | # ''Pseudomonas resinovorans'' polyhydroxyalkanoate synthase (denoted PHA) | + | # ''Pseudomonas resinovorans'' - polyhydroxyalkanoate synthase (denoted PHA) |
<br> | <br> | ||
<br> | <br> | ||
| Line 33: | Line 188: | ||
|- | |- | ||
|style="padding-right: 20px;"|[[File:Step 1 outline.jpg|400px]] | |style="padding-right: 20px;"|[[File:Step 1 outline.jpg|400px]] | ||
| - | |This was our plan in order to validate PLA synthesis. | + | |style="text-align:justify;"|This was our plan in order to validate PLA synthesis. To save money we ordered each of the two heterologous genes in four pieces from IDT giving us 8 GBlocks. An inducible promoter preceded each gene so we could tightly regulate the expression of the enzyme. The terminators were amplified from other sources and given homology to the appropriate Gblocks. |
|} | |} | ||
<br> | <br> | ||
| Line 41: | Line 196: | ||
<center>[[File:GblockPLA.png|800px]]</center><br> | <center>[[File:GblockPLA.png|800px]]</center><br> | ||
<br> | <br> | ||
| - | Each fragment was amplified using PCR | + | Each fragment was amplified using PCR to give homology to adjacent fragments. Using Gibson assembly, our plan was to assemble all 10 fragments into one construct. <br><br> |
<center>[[File:PLAgenes.png|800px]]</center><br> | <center>[[File:PLAgenes.png|800px]]</center><br> | ||
<br> | <br> | ||
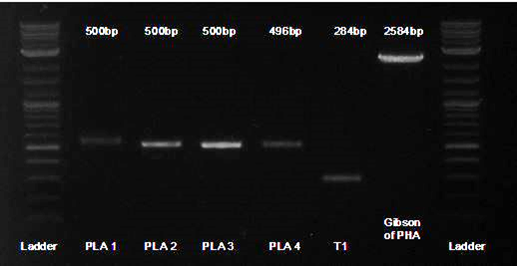
| - | + | To facilitate the process, we used Gibson assembly on each gene separately. Here is a gel of the first 4 Gblocks (labeled PLA1-4), along with the first terminator (labeled T1), and then the 5 pieces assembled together.<br> | |
<br> <center>[[File:PHAgel.JPG|600px]] [[File:PHA.png|200px]]</center><br> | <br> <center>[[File:PHAgel.JPG|600px]] [[File:PHA.png|200px]]</center><br> | ||
| Line 62: | Line 217: | ||
<br> <center>[[File:Seq.zoomed.png|650px]]</center><br> | <br> <center>[[File:Seq.zoomed.png|650px]]</center><br> | ||
| - | *These are the results of the alignment of all 8 fragments with the desired sequence. Ignoring the mismatches at the beginning and end of the sequencing fragments, along with those mismatches covered by the complementary fragment, there appears to only be one legitimate error in our construct. It is denoted below with the red arrow. Fortunately, this happens to fall in a noncoding region of the construct (after the first terminator and before the second promoter). | + | *<p style="text-align:justify;">These are the results of the alignment of all 8 fragments with the desired sequence. Ignoring the mismatches at the beginning and end of the sequencing fragments, along with those mismatches covered by the complementary fragment, there appears to only be one legitimate error in our construct. It is denoted below with the red arrow. Fortunately, this happens to fall in a noncoding region of the construct (after the first terminator and before the second promoter).</p> |
<br> <center>[[File:Wholeseq.png|550px]]</center><br> | <br> <center>[[File:Wholeseq.png|550px]]</center><br> | ||
<br><br> | <br><br> | ||
| - | === | + | === Insertion via Plasmid === |
*In order to insert the two heterologous genes into ''E. coli'', we used Gibson assembly to add our construct onto a plasmid with KanR as a selectable marker. | *In order to insert the two heterologous genes into ''E. coli'', we used Gibson assembly to add our construct onto a plasmid with KanR as a selectable marker. | ||
| - | <center>[[File:PZE plasmid.JPG | + | **Here is the map of the plasmid and the results of the transformation |
| + | **This strain we used in all subsequent steps of the project | ||
| + | <center>[[File:PZE plasmid.JPG]]</center><br> | ||
<center>[[File:EcNR2transformPLA.png|600px]]</center><br> | <center>[[File:EcNR2transformPLA.png|600px]]</center><br> | ||
| Line 75: | Line 232: | ||
==== TolC Negative Selection ==== | ==== TolC Negative Selection ==== | ||
| - | *We used a strain with TolC located at 21B, a highly recombinogenic site in the genome (Isaacs et al. 2011). Our plan was to replace TolC with our construct using double stranded DNA recombination, then use colicin E1 negative selection to pick desired cells (DeVito 2007). | + | *We used a strain with TolC located at 21B, a highly recombinogenic site in the genome (Isaacs et al. 2011). Our plan was to replace TolC with our construct using double stranded DNA recombination, then use colicin E1 negative selection to pick desired cells (DeVito 2007). |
| + | *We had difficulties getting this method to work | ||
<center>[[File:TolCnegative.JPG|400px]]</center><br> | <center>[[File:TolCnegative.JPG|400px]]</center><br> | ||
====Positive Selection with KanR==== | ====Positive Selection with KanR==== | ||
| - | *Since we had trouble with the TolC negative selection, we decided to pursue an alternative option | + | *Since we had trouble with the TolC negative selection, we decided to pursue an alternative option — positive selection. |
**We planned to add kanamycin resistance to the end of our construct and use double-stranded recombination to integrate it into the genome again at site 21B. | **We planned to add kanamycin resistance to the end of our construct and use double-stranded recombination to integrate it into the genome again at site 21B. | ||
| + | **We also had difficulties getting this method to work | ||
| + | **We decided to postpone this step of our experiment and use the strain with our construct on a plasmid inside ''E. coli'' | ||
<center>[[File:KanR positive select.JPG|400px]]</center><br> | <center>[[File:KanR positive select.JPG|400px]]</center><br> | ||
<center>[[File:Screen Shot 2013-08-05 at 10.43.48 AM.png|200px]]</center><br> | <center>[[File:Screen Shot 2013-08-05 at 10.43.48 AM.png|200px]]</center><br> | ||
| + | *Inserting our construct into the genome is still an ongoing process that will hopefully be completed soon | ||
Latest revision as of 00:26, 27 September 2013
Contents |
Engineer strains of E. coli to validate PLA synthesis
Synthesizing and Assembling the Heterologous Enzymes
- In order to reproduce the results of the Lee group we needed to insert the two heterologous genes.
- Clostridum propionicum - propionate CoA transferase (denoted PCT)
- Pseudomonas resinovorans - polyhydroxyalkanoate synthase (denoted PHA)
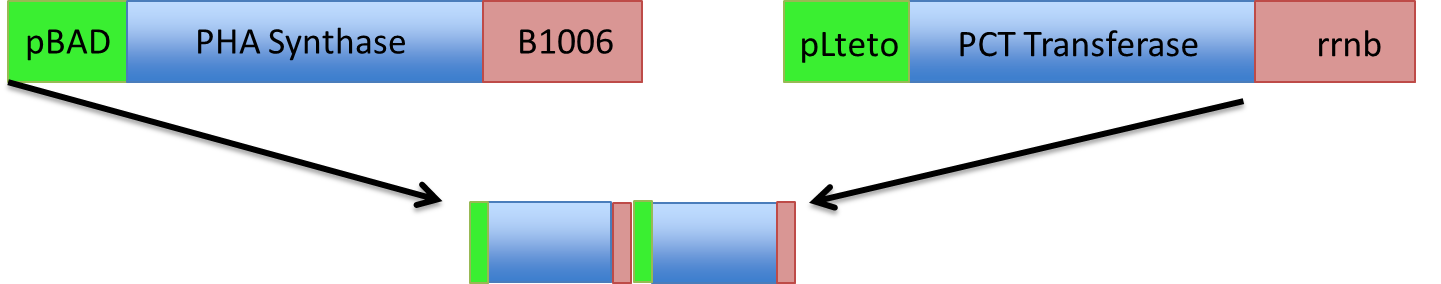
Here is a schematic of what the entire construct looks like with both promoter, genes and terminators.
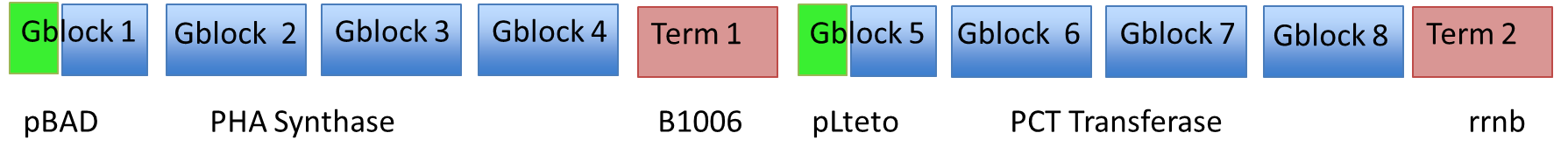
Each fragment was amplified using PCR to give homology to adjacent fragments. Using Gibson assembly, our plan was to assemble all 10 fragments into one construct.

To facilitate the process, we used Gibson assembly on each gene separately. Here is a gel of the first 4 Gblocks (labeled PLA1-4), along with the first terminator (labeled T1), and then the 5 pieces assembled together.

Here is a gel of the assembly of the last 4 Gblocks, along with the second terminator.
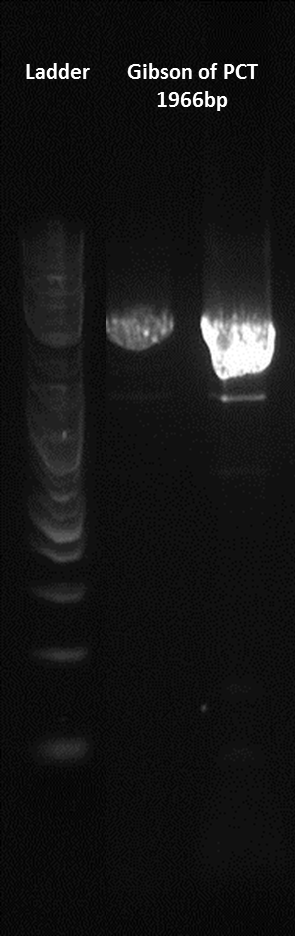

Here is a gel of the assembly of the entire construct.
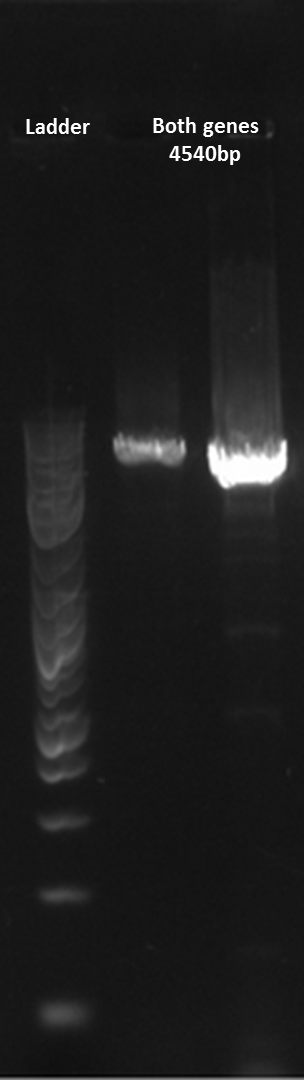
Sequencing the construct
- Keck Biotechnology Resource Laboratory kindly sequenced our construct for us
- Using our Geneious license we aligned the sequencing results with the desired sequence of our construct
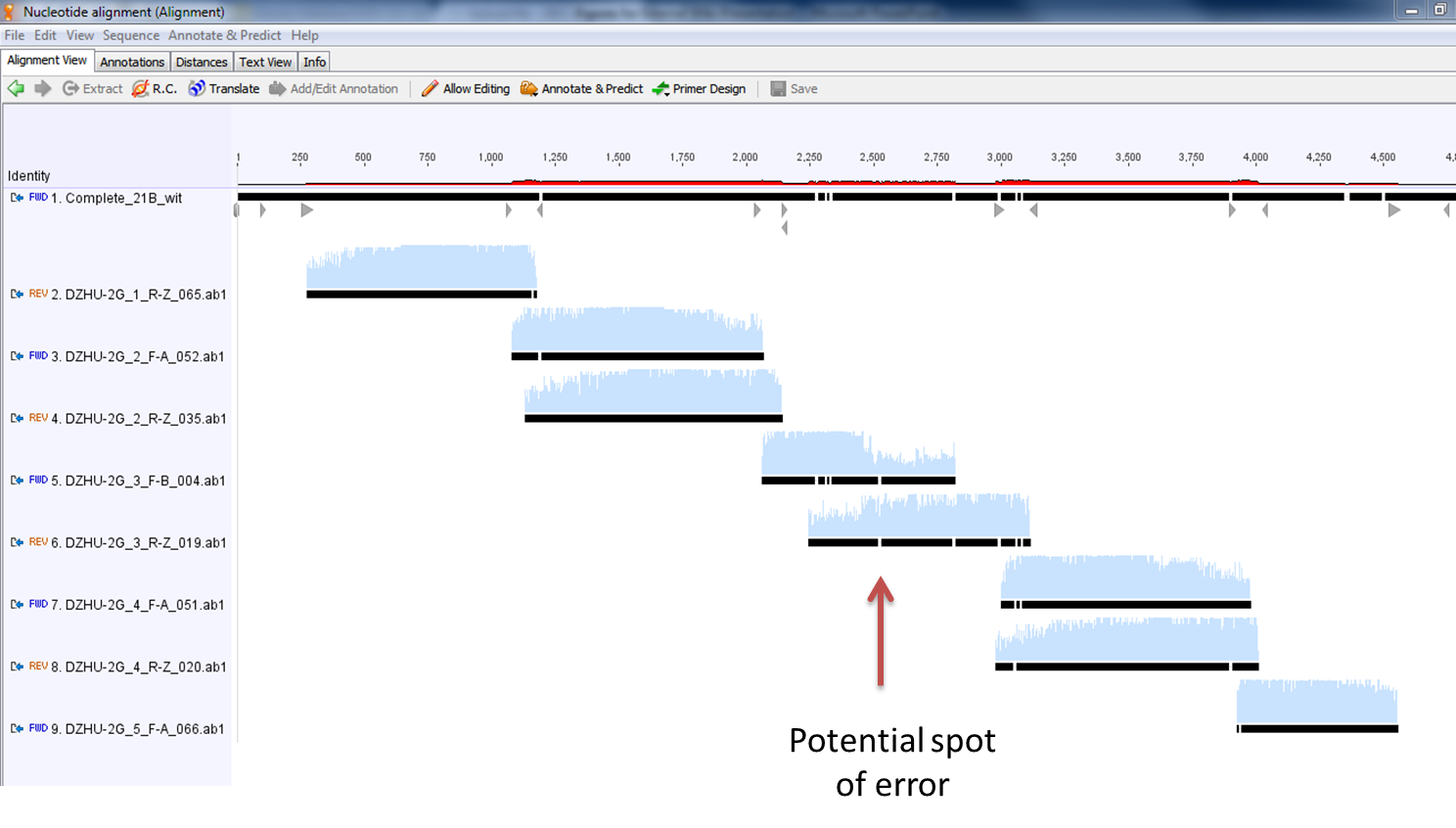
- Here is a zoomed-in picture of the results

These are the results of the alignment of all 8 fragments with the desired sequence. Ignoring the mismatches at the beginning and end of the sequencing fragments, along with those mismatches covered by the complementary fragment, there appears to only be one legitimate error in our construct. It is denoted below with the red arrow. Fortunately, this happens to fall in a noncoding region of the construct (after the first terminator and before the second promoter).
Insertion via Plasmid
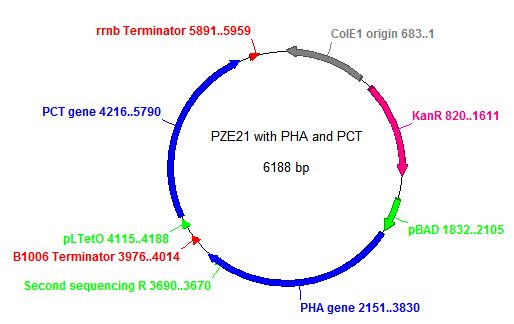
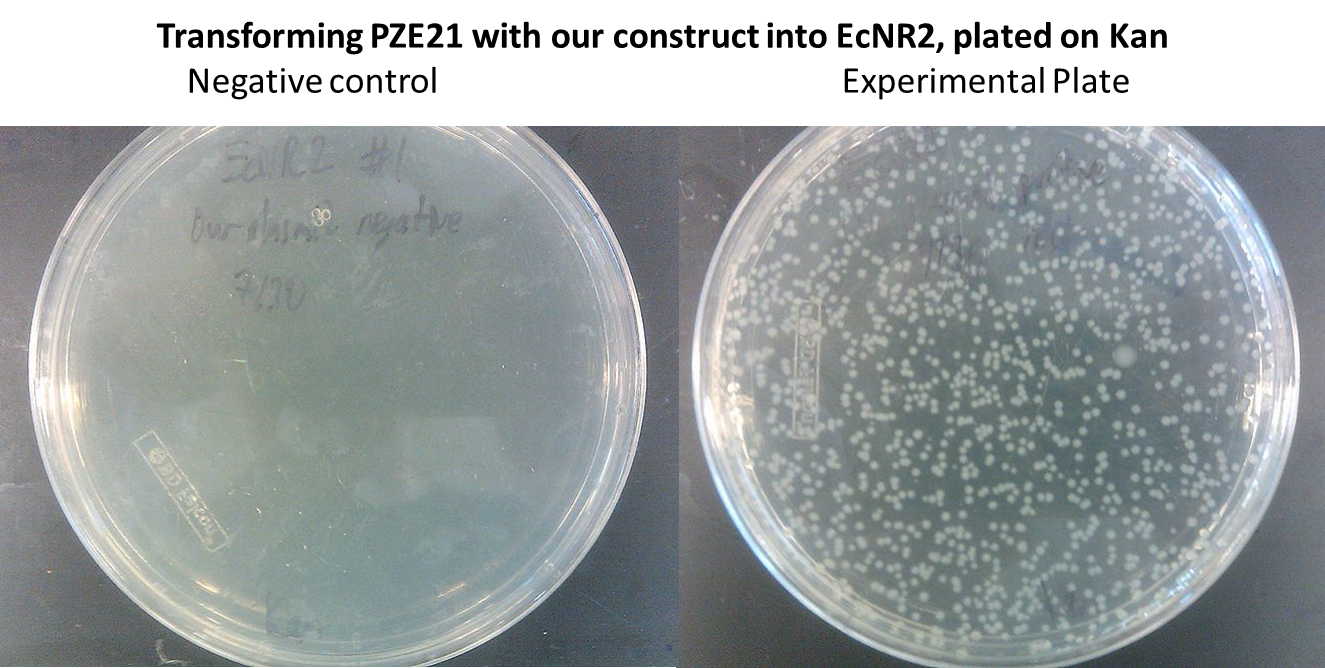
- In order to insert the two heterologous genes into E. coli, we used Gibson assembly to add our construct onto a plasmid with KanR as a selectable marker.
- Here is the map of the plasmid and the results of the transformation
- This strain we used in all subsequent steps of the project
Inserting into the Genome
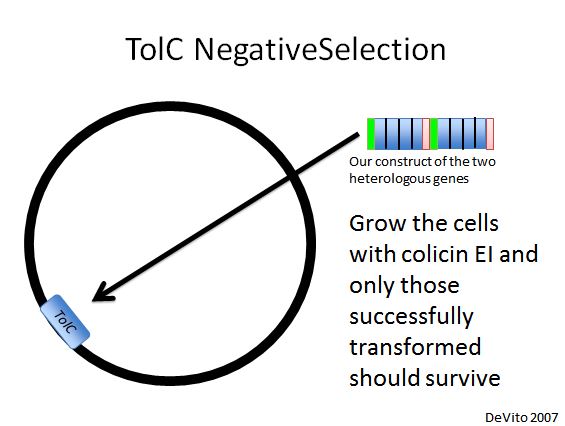
TolC Negative Selection
- We used a strain with TolC located at 21B, a highly recombinogenic site in the genome (Isaacs et al. 2011). Our plan was to replace TolC with our construct using double stranded DNA recombination, then use colicin E1 negative selection to pick desired cells (DeVito 2007).
- We had difficulties getting this method to work
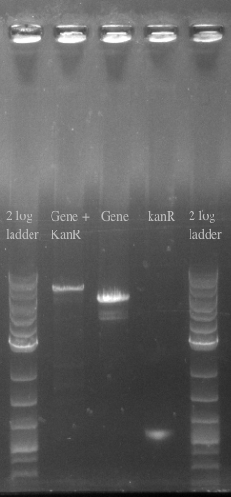
Positive Selection with KanR
- Since we had trouble with the TolC negative selection, we decided to pursue an alternative option — positive selection.
- We planned to add kanamycin resistance to the end of our construct and use double-stranded recombination to integrate it into the genome again at site 21B.
- We also had difficulties getting this method to work
- We decided to postpone this step of our experiment and use the strain with our construct on a plasmid inside E. coli

- Inserting our construct into the genome is still an ongoing process that will hopefully be completed soon
 "
"