Team:UGent/Project
From 2013.igem.org
| Line 16: | Line 16: | ||
<li><i>Segregational instability</i> is caused by unequal distribution of plasmids to daughter cells, which leads to cells devoid of any plasmids. This problem has been solved by using selectable markers (e.g. antibiotic resistance) or post-segregational killing to remove plasmid-lacking cells.</li> | <li><i>Segregational instability</i> is caused by unequal distribution of plasmids to daughter cells, which leads to cells devoid of any plasmids. This problem has been solved by using selectable markers (e.g. antibiotic resistance) or post-segregational killing to remove plasmid-lacking cells.</li> | ||
<li><i>Structural instability</i> leads to an incorrect expression of proteins. This problem finds its cause in plasmids with a changed DNA sequence. However, these mutations occur at a low frequency.</li> | <li><i>Structural instability</i> leads to an incorrect expression of proteins. This problem finds its cause in plasmids with a changed DNA sequence. However, these mutations occur at a low frequency.</li> | ||
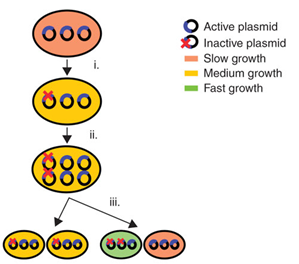
| - | <li><i>Allele segregation</i> up to now remains mainly unaddressed. When a mutation occurs in the gene of interest, but not in the selectable marker, cells can emerge that are resistant to the selection, but not productive. As a result they cannot be removed by the use of selectable markers or post-segregational killing (Figure). After the mutation occurs, the plasmids are replicated and divided over daughters cells. The mutated plasmids can be divided in two different ways: either each daughter cell receives one mutated plasmid, or only one daughter cell receives both. In the latter case, the cell receiving both mutated plasmids produces less of the desired product and grows faster than the other cell. This growth advantage is due to the fact that the new synthetic pathway that has been inserted places a heavy metabolic burden on cells and reduces the cellular fitness. Therefore, cells with mutated plasmids accumulate and lead to a great productivity loss.</li></ul> | + | <table> |
| + | <tr> | ||
| + | <td> | ||
| + | <p><li><i>Allele segregation</i> up to now remains mainly unaddressed. When a mutation occurs in the gene of interest, but not in the selectable marker, cells can emerge that are resistant to the selection, but not productive. As a result they cannot be removed by the use of selectable markers or post-segregational killing (Figure). After the mutation occurs, the plasmids are replicated and divided over daughters cells. The mutated plasmids can be divided in two different ways: either each daughter cell receives one mutated plasmid, or only one daughter cell receives both. In the latter case, the cell receiving both mutated plasmids produces less of the desired product and grows faster than the other cell. This growth advantage is due to the fact that the new synthetic pathway that has been inserted places a heavy metabolic burden on cells and reduces the cellular fitness. Therefore, cells with mutated plasmids accumulate and lead to a great productivity loss.</li></p> | ||
| + | </td> | ||
| + | <td> | ||
| + | </html>[[File:UGent_2013_AlleleSegregation.png|250px|link=]]<html> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> | ||
| + | </table> | ||
| + | |||
| + | </ul> | ||
</p> | </p> | ||
</html> | </html> | ||
Revision as of 09:07, 13 September 2013
|
Introduction: high gene expression in industrial biotechnologyThe main goal of industrial biotechnology is to increase the yield of biochemical products using microorganisms as production hosts. This includes engineering large synthetic pathways and improving their expression. Overexpression of genes has mainly been achieved by using high or medium copy plasmids. However, studies have demonstrated that plasmid-bearing cells lose their productivity fairly quickly as a result of genetic instability.
In industrial biotechnology, a common technique to express new synthetic products and pathways is the use of plasmids as vectors. Plasmids are easy to insert into cells and replicate independently from the genome, allowing strong gene expression. Overexpression is easily achieved by using plasmids with a medium or high copy number, different promoter systems, ribosome binding sites (RBS), etc. Thanks to plasmids, the industrial biotechnology has grown substantially over the past years. However, the use of plasmids entails some important disadvantages: plasmid maintenance imposes a metabolic burden on cells and plasmids suffer from genetic instability. Metabolic burdenWhen plasmids are present in cells and replicate, they create a metabolic burden. This is defined by Bentley et al. (1990) as “the amount of resources that are taken from the host cell metabolism for foreign DNA maintenance and replication”. As a result, metabolic load causes many alterations to the physiology and metabolism of the cell and reduces the cellular fitness. The most common change is a delayed growth. This can be caused by the fact that new pathways for energy generation are activated due to the competition between cell propagation and plasmid replication. This growth retardation leads to a lower yield of the desired product. Genetic instabilityPlasmids are genetically instable due to three processes: segregational instability, structural instability and allele segregation.
Therefore a new method was developed for the overexpression of a gene of interest in the bacterial chromosome: Chemically Inducible Chromosomal evolution (CIChE). In this technique the chromosome is evolved to contain a higher number of gene copies by adding a chemical inducer.
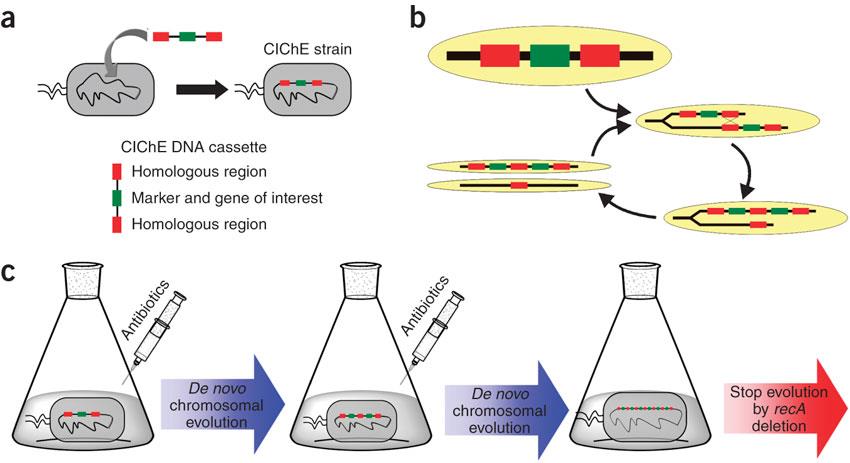
In 2009, Tyo et al. developed a technique for the stable, high copy expression of a gene of interest in E. coli without the use of high copy number plasmids, thus avoiding their previously stated negative characteristics. They called this plasmid-free, high gene copy expression system ‘chemically inducible chromosomal evolution’. In this method, the gene of interest is integrated in the microbial genome and then amplified to achieve multiple copies and reach the desired expression level. Genomic integration guarantees ordered inheritance, resolving the problem of allele segregation. CIChE works as follows: First a construct, containing the gene(s) of interest and the antibiotic marker chloramphenicol acetyl transferase (cat) flanked by homologous regions, is delivered to and subsequently integrated into the ''E. coli'' genome. The construct can be amplified in the genome through tandem gene duplication by recA homologous recombination. Then the strain is cultured in increasing concentrations of chloramphenicol, providing a growth advantage for cells with increased repeats of the construct and thereby selecting for bacteria with a higher gene copy number (Figure).
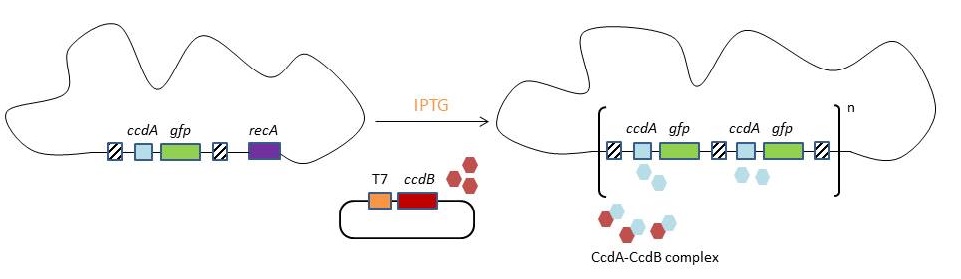
This process is called chromosomal evolution. When the desired gene copy number is reached, recA is deleted, thereby fixing the copy number. CloseThe original model for CIChE, however, results in bacterial strains containing a large number of antibiotic resistance genes. To make this valuable technique more widely applicable in the industry, we developed a model for chromosomal evolution based on a toxin-antitoxin system instead of antibiotic resistance. | |||
 "
"