Team:Tokyo Tech/Project/M13 Shuriken
From 2013.igem.org
| Line 48: | Line 48: | ||
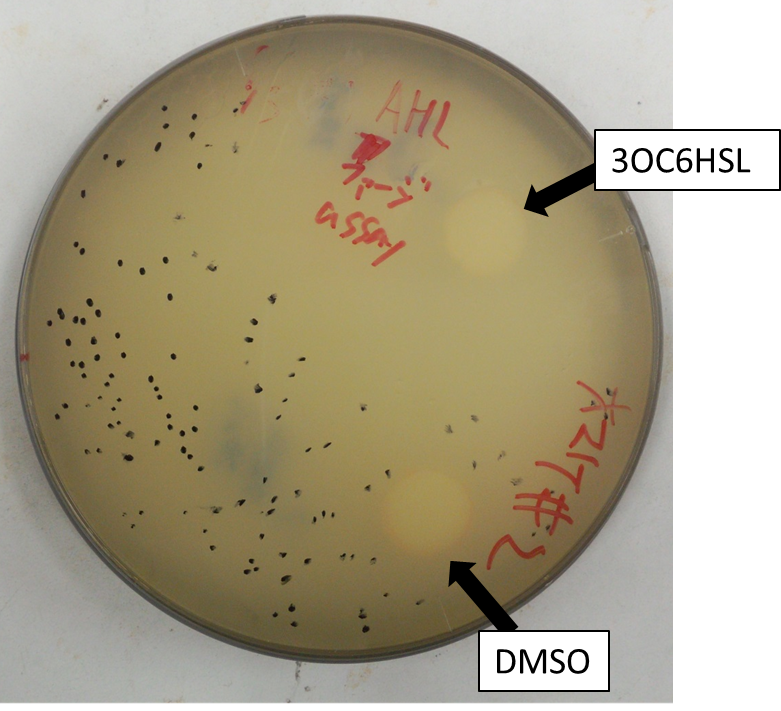
[[Image:Titech2013_Project_M13_shuriken_Fig_2-2-5-A.png|600px|thumb|left|Fig. 4 A. Conclusion that AHL included filter paper induced phage release.]] | [[Image:Titech2013_Project_M13_shuriken_Fig_2-2-5-A.png|600px|thumb|left|Fig. 4 A. Conclusion that AHL included filter paper induced phage release.]] | ||
[[Image:Titech2013_Project_M13_shuriken_Fig_2-2-5-B.png|600px|thumb|right|Fig. 4 B. Conclusion that AHL included filter paper induced phage release.]] | [[Image:Titech2013_Project_M13_shuriken_Fig_2-2-5-B.png|600px|thumb|right|Fig. 4 B. Conclusion that AHL included filter paper induced phage release.]] | ||
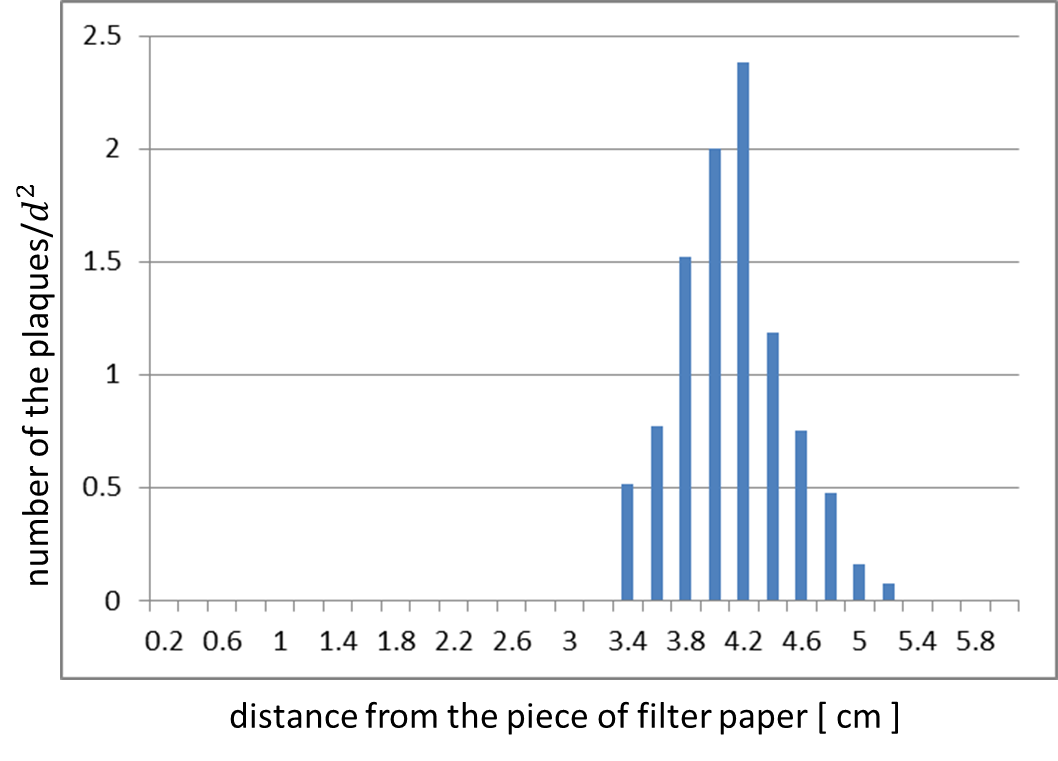
| - | [[Image: | + | [[Image:Titech2013_Project_M13_shuriken_Fig_2-2-6.png|600px|thumb|center|Fig. 5. The distribution histogram of the plaques]] |
</div><br> | </div><br> | ||
Revision as of 13:18, 26 September 2013
Contents |
Abstract
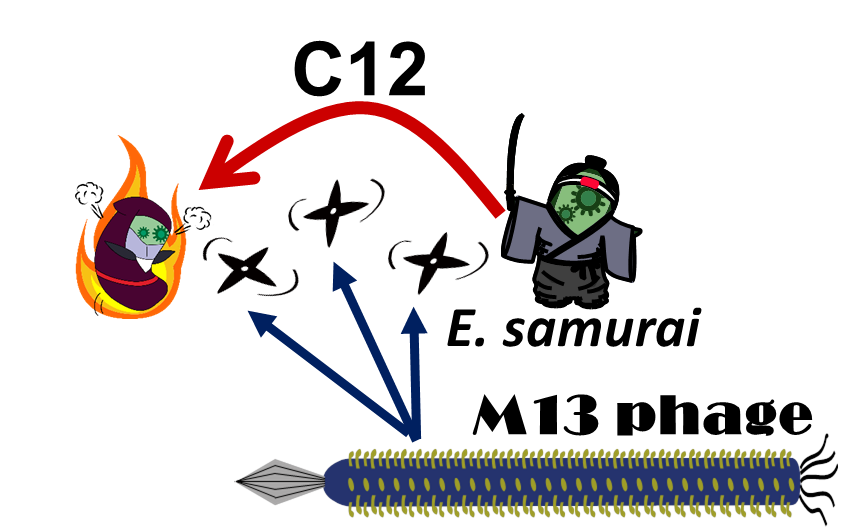
E. ninja throws "shuriken" to attack E. samurai in response to 3OC12HSL, an intercellular molecule. In our story, M13 phages are the shuriken. We confirmed that the system of AHL-dependent release and infection of M13 phage shuriken properly worked, using plaque forming assay.
Phage life cycle dependent on g2p expression
In natural M13 phage life cycle, g2p expression is required for production of a single stranded DNA (ssDNA) and thus required for phage release. Nicking on a double stranded DNA by G2p nickase triggers a series of reactions to amplify the phage ssDNA. In the presence of the ssDNA, the coat proteins are assembled around it. [1][2] (See more...)
Inducible M13 phage release
in the whole circuit design
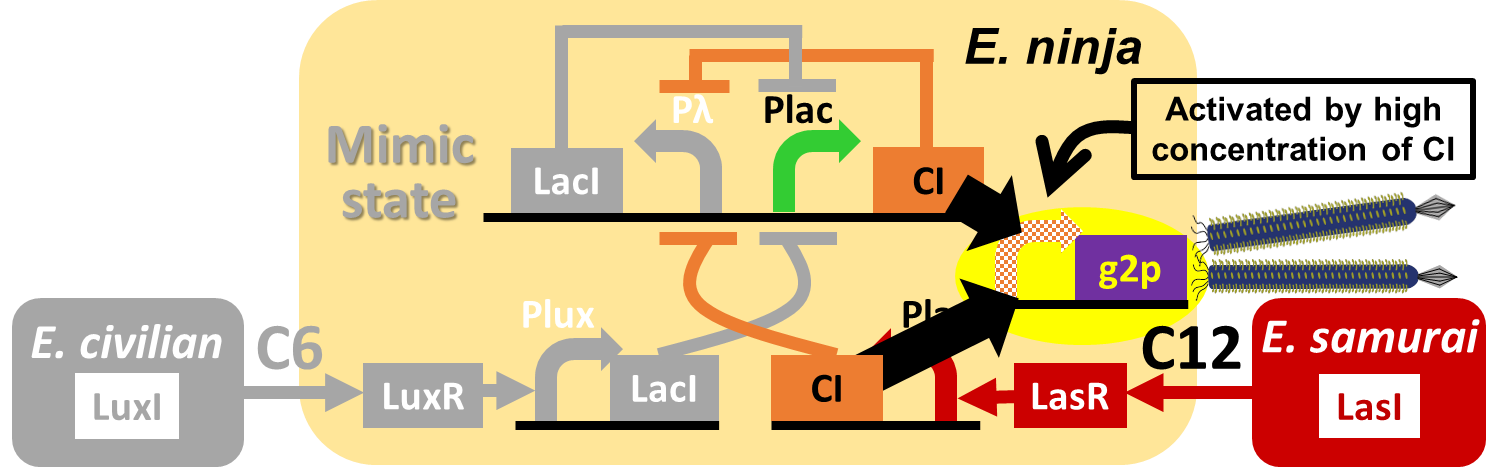
In our story, E. ninja releases the phage particles, only when E. ninja is in the Attack state induced by 3OC12HSL. In the whole circuit (Fig. 2-2-1), g2p expression is indirectly regulated by 3OC12HSL via cI expression.
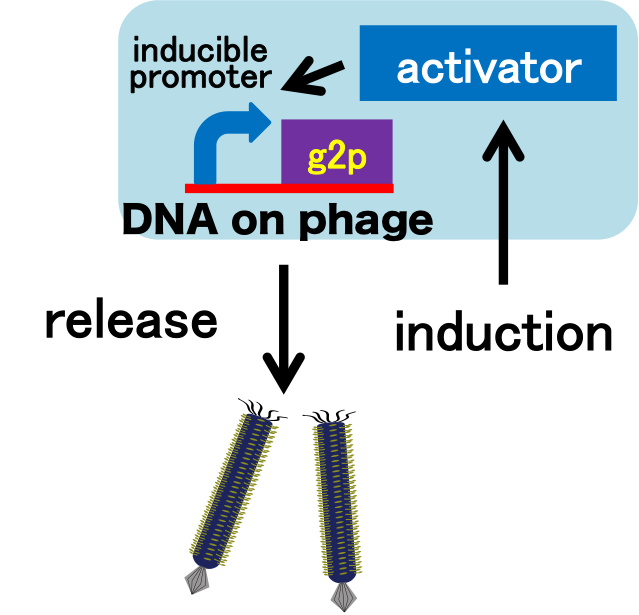
Model system for inducible phage release
We constructed a model system for inducible phage release by regulation of g2p expression. Genome DNA of this engineered phage, shown in Fig. 2-2-2, needs two functions. One is inducible expression of g2p protein. We thus designed to replace the promoter for g2p protein with lux promoter. Note that we used 3OC6HSL, not 3OC12HSL , in this model experiment. The other is maintenance of the genome DNA in the absence of g2p expression. We combined M13 genome double stranded DNA with pSB3K3 backbone.
Firstly, we confirmed that M13 genome with two modifications related to our design kept plaque forming activity. One is replacement of the promoter for g2p protein with a constitutive promoter, PLacIq(BBa_I14032). The other is accommodation of pSB3K3 backbone. Even though the plasmid has two different types of replication origins, M13 origin and pSB3 origin, this plasmid (BBa_K1139020) formed plaque. In contrast, construction intermediates without a promoter upstream of g2p coding sequence (Promoterless-M13 + Plac: BBa_K1139018, Promoterless-M13 + Plac-GFP: BBa_K1139022) could not form plaque. (See more...)
We then confirmed that replacement of the g2p promoter with lux promoter accomplished inducible phage release. For a plaque forming assay, we used a plasmid with lux promoter upstream of g2p coding sequence (Plux-M13-Plac-GFP: BBa_1139021). Besides, as lawn, we used JM109 (F+ strain) which has a plasmid that is luxR+. Also, to make a concentration gradient of the inducer (AHL), we put a piece of filter paper on which dropped the inducer solution.
The result shows that the plaques are formed only when the inducer exists in the medium (Fig. 4). In addition, the distribution of the plaques has an optimum value, depending on the distance from the piece of filter paper which has soaked up the inducer (Fig. 5).
In the neighborhood of the piece of filter paper, the expression of g2p is so activated that the phage particles cannot be produced efficiently, because the production of the coat proteins largely exceeds that of the single stranded DNA, and vice versa.
This result shows that the inducible release of M13 phage is realized; our plan was achieved.
Application
We achieved the DNA messaging system using M13 phage which is released by promoter induction. You can apply our DNA messaging system to following two ideas because you utilize various stimuli for induction.
Drew Endy’s group reported they achieved the cell-cell communication system in which DNA is regarded as message. [3] In this system, phages, which transmit DNA to receiver cell, were released by induction of a helper phage. On the other hand, if you apply our DNA messaging system, you can make DNA messaging system more versatile and complex because you utilize various stimulus, not limiting the inducer of phage release to a helper phage. [4]
It is important to research infectious spectrum, what kinds of bacteria phage can infect, before we use phage for bioremediation. We can use M13 phage as carrier of DNA transfection for bioremediation. If you use M13 phage for transfecting to bacteria living in the environment, we need to research what kinds of bacteria can be the host of M13 phage using a sample of the soil. The gene coding fluorescence protein in the engineered phage we designed is useful when you research the host of M13 phage. When M13 phage including the genetic circuit cording fluorescence protein infects bacteria, they fluoresce. Because the fluorescence intensity depends on the kind of infected cell, you can separate these kinds of bacteria by cell sorter. Also, you can analyze the genome of infected bacteria by single cell genomics. As a similar study, Nojiri’s group and Shintani’s group reported that they can transfect DNA to uncultivable bacteria in soil. [5][6] They use conjugation for transfection of DNA in this research. You can make the idea more versatile by applying our idea because you can also transfect DNA to bacteria which can be the host of M13 phage. Moreover, you could identify the strain which would be the host of M13 phage only on a specific condition because you can control the phage release with knowledge of synthetic biology. For example, if you could find the strain which fluoresces only when AHL exists, the strain would be the host on the condition that AHL exists.
Reference
[1]Atsushi Higashitani. Nahoko Higashitani. Kensuke Horiuchi, DNA Replication in Filamentous Bacteriophage, Protein, Nucleic Acid and Enzyme(1994), vol39:2189-2197.
[2]Kensuke Horiuchi, Origin of DNA replication of filamentous coliphages, Jpn. J. Genet(1990), vol65:225-241.
[3]ME Ortiz. D Endy., Engineered cell-cell communication via DNA messaging, J. Biol. Engineering(2012), 6-16.
[4]A Goñi-Moreno, M Amos, F de la Cruz, Multicellular Computing Using Conjugation for Wiring, PLOS ONE(2013), Vol8.:Issue6:e65986.
[5]M Shintani. N Fukushima. M Tezuka. H Yamane. H Nojiri, Conjugative transfer of the IncP-7 carbazole degradative plasmid, pCAR1, in river water samples, Biotechnol Lett (2008). vol30:117-122
[6]H Nojiri Structural and Molecular Genetic Analyses of the Bacterial Carbazole Degradation System Biosci, Biotechnol, Biochem, 76(1), 1-18, 2012
 "
"