Team:Imperial College/mainresults
From 2013.igem.org
| Line 41: | Line 41: | ||
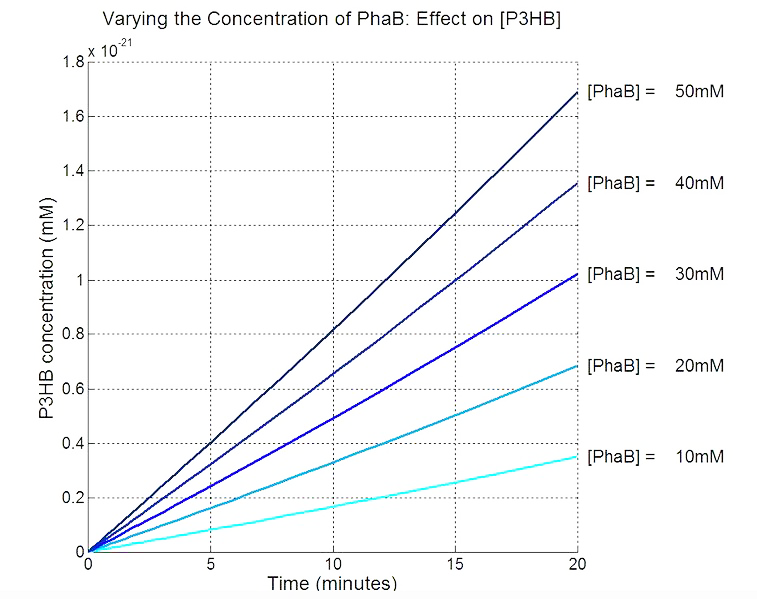
[[File:Imperial College PhaB scan Single cellmM.JPG|thumbnail|center|700px|<b>PhaB concentration in the chassis is a rate limiting factor in P3HB synthesised.</b> The parameter values are from various sources, thus the simulation result should be interpreted as a qualitative perspective and the trend should be observed.]] | [[File:Imperial College PhaB scan Single cellmM.JPG|thumbnail|center|700px|<b>PhaB concentration in the chassis is a rate limiting factor in P3HB synthesised.</b> The parameter values are from various sources, thus the simulation result should be interpreted as a qualitative perspective and the trend should be observed.]] | ||
| - | + | <html> | |
| - | + | <iframe width="960" height="720" src="//www.youtube.com/embed/m2ZJUA-yw34" frameborder="0" allowfullscreen></iframe> | |
| + | </html> | ||
<html> | <html> | ||
</div> | </div> | ||
Revision as of 23:38, 4 October 2013
Main Results
Resource-full Waste
Plastic Fantastic
We made P3HB bioplastic ▼
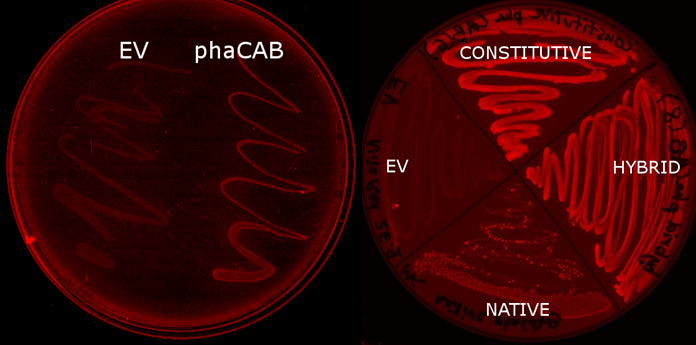
We used E. coli that expresses PhaCAB enzymes to make P3HB bioplastic. P3HB produced in cells were stained with Nile Red for fluorescent microscope imaging, and strong fluorescence was detected at the area where PhaCAB-expressing cells were streaked.
Our model predicts increased PhaB expression boosts P3HB production ▼
In order to realistically improve our experimental design, we produced P3HB synthesis model with considerations of central metabolic pathway within the cells. Our model predicts that during the synthesis of P3HB, the concentration of PhaB enzymes is an important rate limiting factor that can actually be regulated.
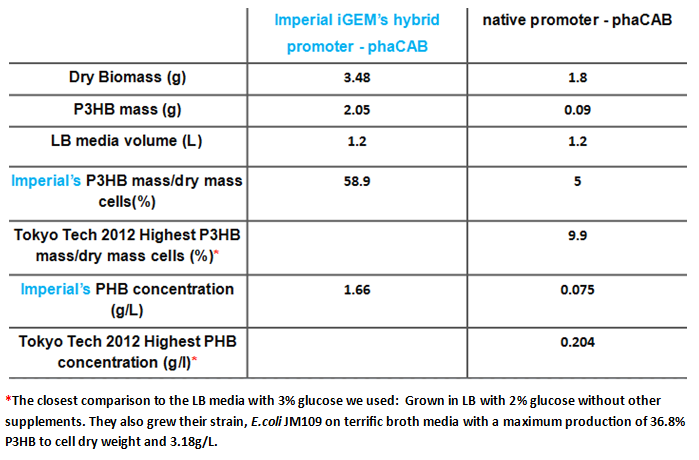
We optimised P3HB bioplastic production ▼
Since our model predicts that P3HB production can be significantly improved by expressing more P3HB polymerase PhaB, we designed a [http://parts.igem.org/Part:BBa_K1149051 hybrid promoter] which, consists of the J23104 constitutive promoter and the native promoter to optimise gene expression. Our results show that we have successfully produced 11-fold more P3HB bioplastic compared with the native promoter.
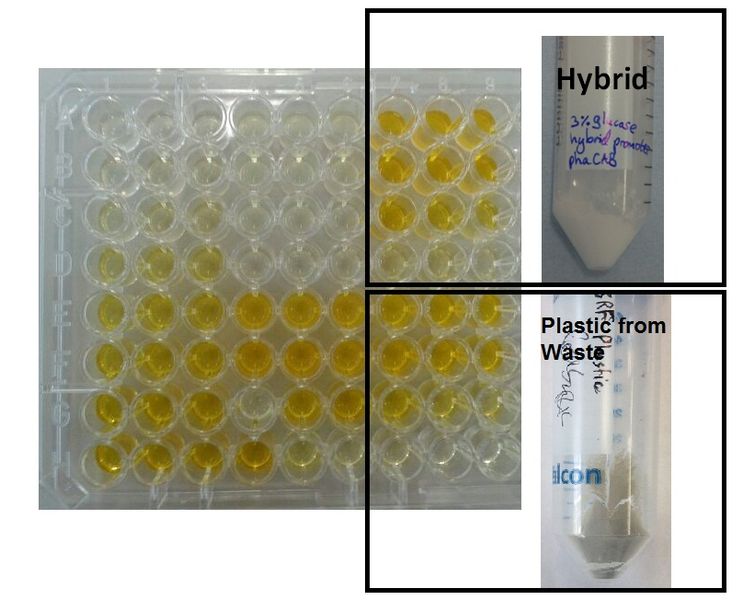
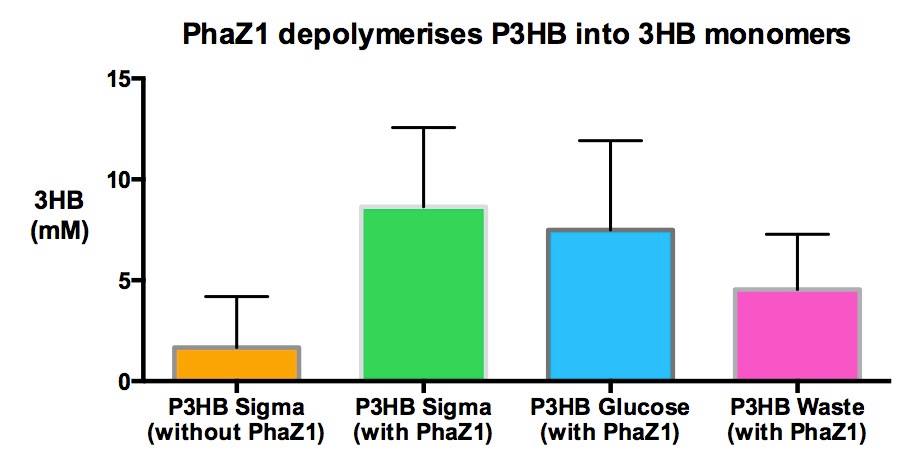
We made bioplastic from mixed waste ▼
One of the objectives of Module 1 is to produce P3HB bioplastic from waste. By comparing degradation product 3HB of P3HB bought from Sigma, produced from glucose and produced from the waste, we found there is no significant differences in 3HB concentration between these samples.
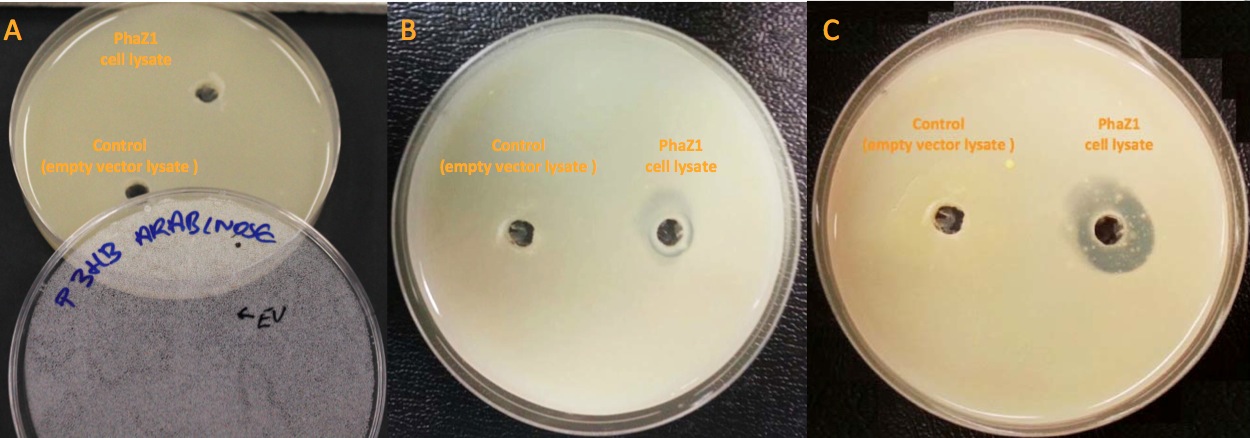
We degraded P3HB ▼
Our clearing zone assay indicates that P3HB depolymerase PhaZ1 started to fairly quickly degrade P3HB from the first day. After 3 days, there is evidently a clear zone around the well containing PhaZ1. We are the first iGEM team to degrade bioplastics!
We degraded P3HB we made from waste ▼
Using 3HB colourimetric assay kit, we have shown that we have degraded the P3HB made from waste into 3HB monomers. In addition, there is no significant difference in 3HB concentration between different P3HB sources. This result proves that we now have a closed loop for P3HB bioplastic recycling!
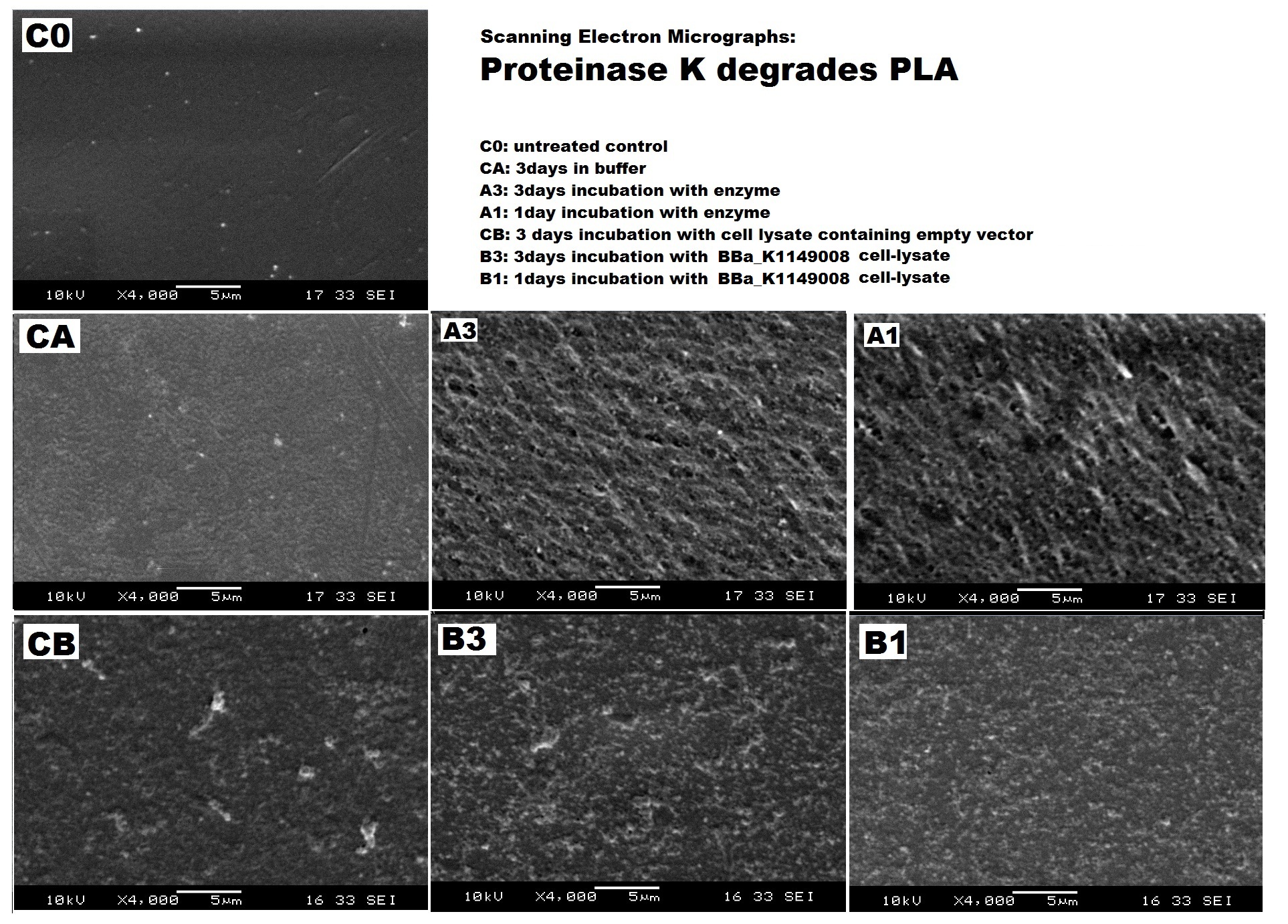
We degraded PLA ▼
 "
"