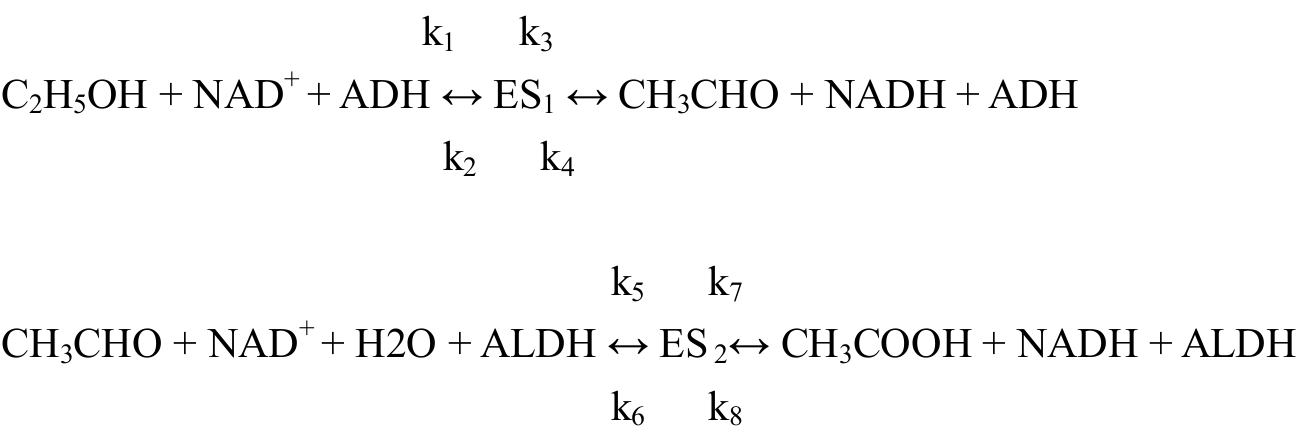Team:CAU China/Model
From 2013.igem.org
| Line 103: | Line 103: | ||
[[File:cau_chinamodela.jpg]] | [[File:cau_chinamodela.jpg]] | ||
'''According to the law of mass action, we can get:''' | '''According to the law of mass action, we can get:''' | ||
| - | + | [[File:cau_chinamodelb]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
'''And according to the law of conversation of mass, there are another four equations:''' | '''And according to the law of conversation of mass, there are another four equations:''' | ||
| - | + | [[File:cau_chinamoedlc]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Because of the gastric motility, the maximum of time is 4 hours. And the volume of gastric contents is about 1L, the value of a half full adult. Because we aim to improve the enzyme activity at pH2.0 to a natural or higher level, so the k1to k8 are cited from the reported data[1][2]. The data are got at the optimal conditions. | Because of the gastric motility, the maximum of time is 4 hours. And the volume of gastric contents is about 1L, the value of a half full adult. Because we aim to improve the enzyme activity at pH2.0 to a natural or higher level, so the k1to k8 are cited from the reported data[1][2]. The data are got at the optimal conditions. | ||
Revision as of 16:06, 26 September 2013

|
1 Project descriptionWe try to design a kind of beverage, containing engineered strains, to resist alcohol. Alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase (ALDH) can be expressed in the strains and after lysis, the two enzymes can transfer ethanol into acetic acid in the stomach. Then drunkenness can be relieved. This model aims at describing how the concentration of substrates changing under the function of our enzymes. And an instruction is given to help consumers to decide how much beverage they need according how much alcohol they drink. 2 ModelWe mainly build a group of enzyme kinetic equations to describe the enzyme catalysis reactions. With this model, we can know how much ethanol can be transferred successfully with a certain amount of enzymes. 2.1 Assumptions1 Consumers should drink the alcohol-toxic beverage firstly and then drink alcohol. 2 The enzymes can finish their work before gastric emptying. 3 The frequency of alcohol intake and the amount of each time are not consider. 4 The combination of ADH and ALDH can catalyze the reaction in a most efficient way. 5 After the carrier strains lysis, the total amount of enzymes stay constant. 6 The pH of the reaction system fluctuates around pH2.0. 7 The reaction volume stays constant. 2.2 Symbol description
2.3 Main body of the modelAccording to the structure of the ADH and ALDH, there is very likely an equilibrium state. The enzymes and substrates combine into an equilibrium state, called ES, and then turn into the products. These processes can be shown as the following functions.
2.4 Model solutionWe mainly use Matlab to solve the ordinary differential equations. The analysis is based on the Runge-Kutta method of 4 step algorithm. A bottle of 75cl wine contains about 1.32mol ethanol. With the activity of 0.02uM ADH and 0.4uM ALDH, the concentrations of each substrate can be shown as figures. We can see that the ethanol can be degraded within about one minute, which indicates the effectiveness of our beverage.
3 Instruction of the beverageConsumers should drink the alcohol-toxic beverage firstly and then drink alcohol. The time interval should be no longer than 4 hours and the shorter, the better. According to the results of the model, consumers can choose how much beverage they need according to how much alcohol they drink.
Reference[1] Zhao Q, Hou Y, Gong G H, et al. Characterization of Alcohol Dehydrogenase from Permeabilized Brewer's Yeast Cells Immobilized on the Derived Attapulgite Nanofibers[J]. Applied biochemistry and biotechnology, 2010, 160(8): 2287-2299. [2] Li X, Li Y, Wei D, et al. Characterization of a broad-range aldehyde dehydrogenase involved in alkane degradation in Geobacillus thermodenitrificans NG80-2[J]. Microbiological research, 2010, 165(8): 706-712. |
 "
"