Team:USTC CHINA/Project/Results
From 2013.igem.org
(Difference between revisions)
| Line 74: | Line 74: | ||
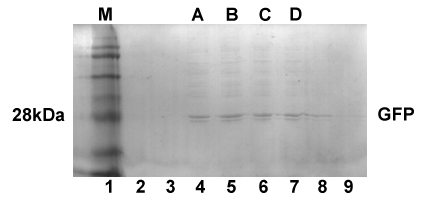
<p>Using GFP to prove the validity of a newly designed circuit is a classical way to verify the expressing of this circuit. As expressions in E.coli involve neither secretory nor sequential problems, we hoped to verify the practicality of our locus by the expression of TD1-GFP. Thus we selected pET22b,which is a common recombinant vector for plasmid construction, as our recombinant vector and E.coli BL21 as engineered bacteria . We fused sequence TD1-GFP with T7 promoter from pET22b downstream and succeeded in expressing fusion protein TD1-GFP, induced by IPTG. </p> | <p>Using GFP to prove the validity of a newly designed circuit is a classical way to verify the expressing of this circuit. As expressions in E.coli involve neither secretory nor sequential problems, we hoped to verify the practicality of our locus by the expression of TD1-GFP. Thus we selected pET22b,which is a common recombinant vector for plasmid construction, as our recombinant vector and E.coli BL21 as engineered bacteria . We fused sequence TD1-GFP with T7 promoter from pET22b downstream and succeeded in expressing fusion protein TD1-GFP, induced by IPTG. </p> | ||
<img src="https://static.igem.org/mediawiki/2013/d/d7/2013ustc-chinajiaotuTD1-GFP.jpg"> | <img src="https://static.igem.org/mediawiki/2013/d/d7/2013ustc-chinajiaotuTD1-GFP.jpg"> | ||
| - | < | + | <div class="atfigure" align="center">Fig1. SDS PAGE shows the molecule weight of TD1-GFP</div> |
<div style="float:left;width:290px;"> | <div style="float:left;width:290px;"> | ||
<img src="https://static.igem.org/mediawiki/igem.org/2/20/2013ustc-china_ygt%28GFP%29.png" width="290" height="290"/> | <img src="https://static.igem.org/mediawiki/igem.org/2/20/2013ustc-china_ygt%28GFP%29.png" width="290" height="290"/> | ||
| Line 81: | Line 81: | ||
<img src="https://static.igem.org/mediawiki/igem.org/f/f9/2013ustc-china_blank.png" width="290" height="290"/> | <img src="https://static.igem.org/mediawiki/igem.org/f/f9/2013ustc-china_blank.png" width="290" height="290"/> | ||
</div> | </div> | ||
| + | <div class="atfigure" align="center">A. BL21 colony Induced by IPTG; B. BL21 colony without IPTG<br> | ||
| + | Fig2. The expression of GFP under fluorescence microscope </div> | ||
| + | |||
</div> | </div> | ||
| - | + | ||
<div><p>② The expression of recombinant antigen and adjuvant in E.coli BL21.</p></div> | <div><p>② The expression of recombinant antigen and adjuvant in E.coli BL21.</p></div> | ||
<img src="https://static.igem.org/mediawiki/2013/5/50"/> | <img src="https://static.igem.org/mediawiki/2013/5/50"/> | ||
Revision as of 21:09, 26 September 2013
 "
"