Team:Yale/Project MAGE
From 2013.igem.org
(Difference between revisions)
(→Results) |
(→Results) |
||
| Line 254: | Line 254: | ||
{| | {| | ||
|- | |- | ||
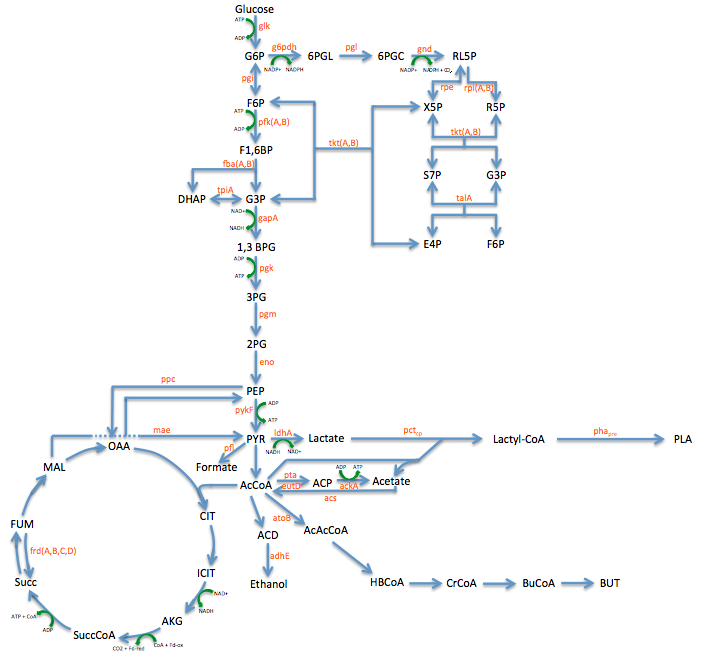
| - | | | + | |The first sample is the baseline. This is EcNR2 with our plasmid containing both the PCT and PHA gene. The strain was grown overnight with the cells induced and in the presence of Nile red to strain the PLA. The gate (labeled P2) was chosen to select those with the highest levels of fluorescence. Due to the abnormally high levels of fluorescence in some cells in the baseline, the gate was chosen to include some baseline cells but only those with very high levels of fluorescence. |
|style="padding-left: 20px; padding-right: 20px;"|[[File:Baseline both.jpg|400px]] | |style="padding-left: 20px; padding-right: 20px;"|[[File:Baseline both.jpg|400px]] | ||
|} | |} | ||
| + | |||
| + | ---- | ||
| + | |||
{| | {| | ||
|- | |- | ||
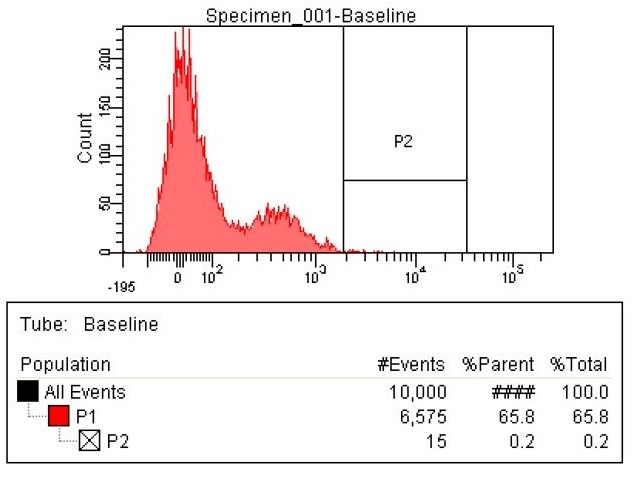
| - | | | + | |The second sample is the KO oligos. This means it has 11 Oligos to knock out 11 enzymes. The enzymes targeted for Knockout were ackA, frdB, frdD pflA, pflB, adhE, frdA, frdC, ATOB, PTA, EUTD. There are now 26 cells within the P2 gate compared to the 15 in the baseline. That means number of cells with high levels of fluorescence nearly doubled. |
|style="padding-left: 20px; padding-right: 20px;"|[[File:64-KO knockouts both.jpg|400px]] | |style="padding-left: 20px; padding-right: 20px;"|[[File:64-KO knockouts both.jpg|400px]] | ||
|} | |} | ||
| + | |||
| + | ---- | ||
| + | |||
{| | {| | ||
|- | |- | ||
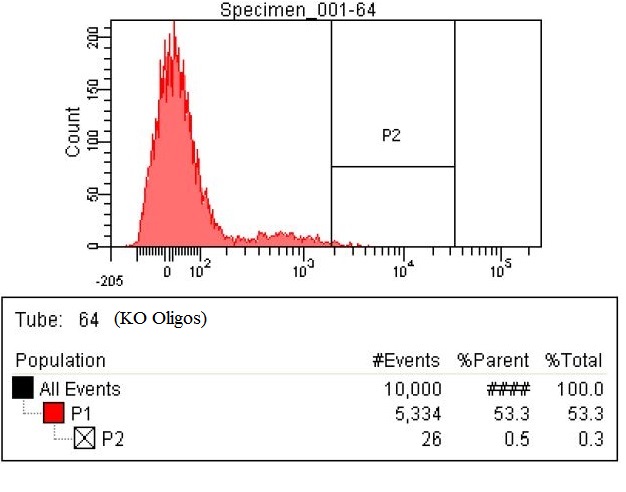
| - | | | + | |The third sample is the RBS tuning Olgios. These are oligos with degenerate RBS with the following sequence DDRRRRRDDDD (-4 through -14 positions from the start codon). The enzymes targeted for RBS tuning are DHA, ACS, ATOB, EUTD, PTA, MAEA, MAEB, PYKF, ENO, PGM, PGK, GAPA, TPIA, FBAA, FBAB, PFKA, PFKB, GLK, PGL, RPOS. The number of cells within the P2 gate is 65 which is greater than a fourfold increase. |
|style="padding-left: 20px; padding-right: 20px;"|[[File:64-RBS both.jpg|400px]] | |style="padding-left: 20px; padding-right: 20px;"|[[File:64-RBS both.jpg|400px]] | ||
|} | |} | ||
| + | |||
| + | ---- | ||
| + | |||
| + | {| | ||
| + | |- | ||
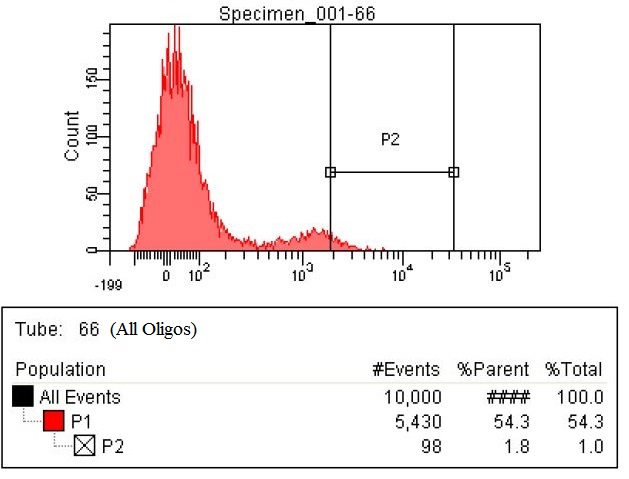
| + | |The fourth sample is the all Olgios. This is a combination of both the RBS oligos and the KO oligios. The number of cells within the P2 gate is 65 which is greater than a fourfold increase. This sample has 98 cells within the gate, which is a six and a half fold increase. | ||
| + | |style="padding-left: 20px; padding-right: 20px;"|[[File:66-All both.jpg|400px]] | ||
| + | |} | ||
| + | |||
| + | ---- | ||
| + | |||
| + | {| | ||
| + | |- | ||
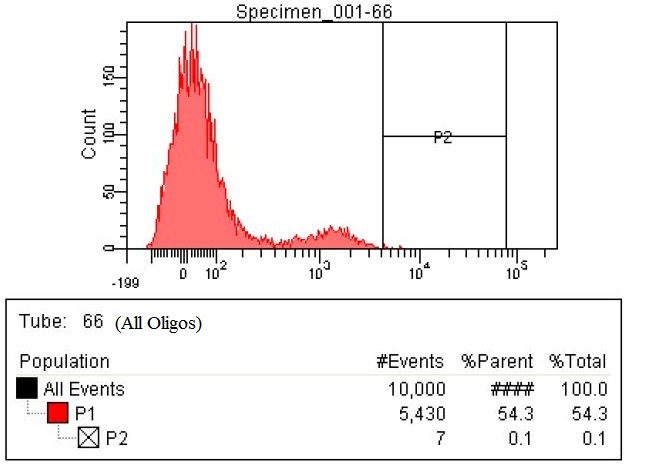
| + | |Due to the fact the last sample with all the oligos had so many cells within the gate we decided to make a stringent gate. This gate was moved to only select the top 0.1% of the cells. This corresponded to 7/10,000. These cells were collected as well as the cells from the three previous graphs | ||
| + | |style="padding-left: 20px; padding-right: 20px;"|[[File:66-All high both.jpg|400px]] | ||
| + | |} | ||
| + | |||
| + | === Next steps === | ||
| + | *The next step is to grow up these cultures (we sorted our 1,000 cells each time to start a culture). | ||
| + | *These cells will be tested on the plate reader compared to the wild type EcNR2, as well as the EcNR2 with our plasmid. There should be an increase in levels of fluorescence, due to increased PLA production. With only 1 MAGE cycles we were able generate a twofold increase, so in theory with an additional 5 MAGE cycles this increase will be even more drastic. | ||
| + | *This experiment will be done within the next week and the results will be presented at the Regional Competition | ||
===== List of Papers: ===== | ===== List of Papers: ===== | ||
Revision as of 22:44, 27 September 2013
Contents |
MAGE Targets
- The first step in applying MAGE is finding MAGE targets. This involved reading numerous scientific papers to learn as much as possible about the heterologous enzymes, and the pathway that was being used to create the PLA
Enzyme Targets
- Sadly there was no crystal structure of either enzyme we could use to locate the sites to introduce mutations
- However, we used the literature available to locate spots where we would want to introduce mutations
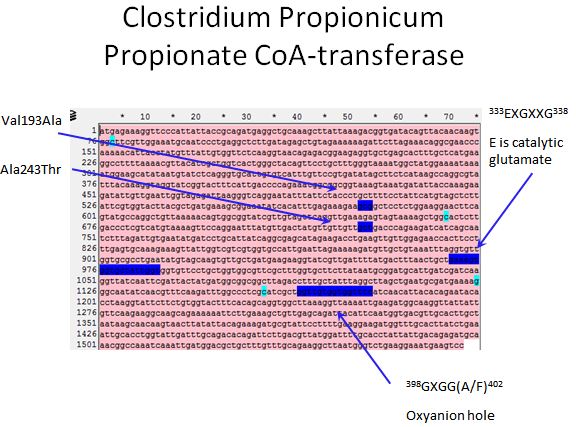
Propionate CoA-transferase
| 
|
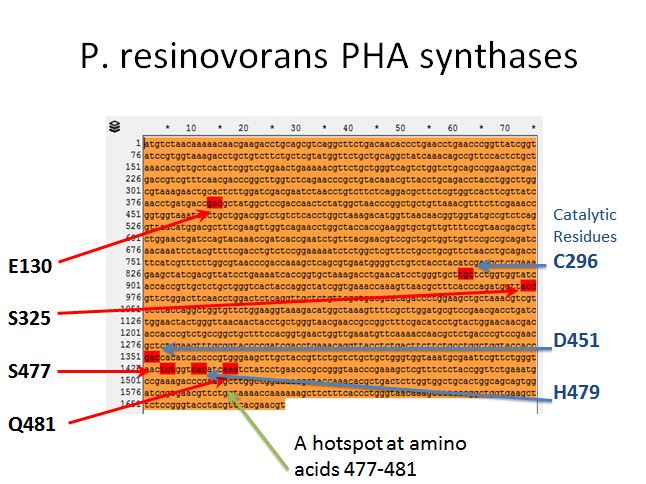
P. resinovorans PHA synthases
| 
|
Pathway Engineering
- We wanted to divert resources toward our desired pathway
- This mainly consisted of increasing the production of lactate
- In order to better understand the pathway we were tampering with we created this metabolic engineering graphic (using the sources listed at the bottom of this page)
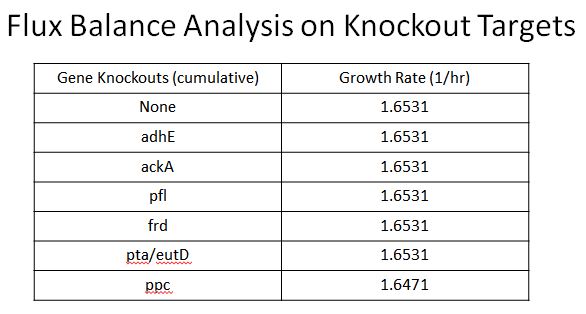
Enzyme KOs
| 
|
RBS Tuning
| 
|
Results
- The first time we used FACS to sort the cells, we saw roughly a two fold increase in fluorescence when we tested on the plate reader
- This was sorting after a single MAGE cycle with the KO olgios
- We used this FACS sorted strain and ran 5 more MAGE cycles (one with RBS oligos, one with KO, and one with all Oligos)
- Here are the results of the FACS sorting of these strains
Next steps
- The next step is to grow up these cultures (we sorted our 1,000 cells each time to start a culture).
- These cells will be tested on the plate reader compared to the wild type EcNR2, as well as the EcNR2 with our plasmid. There should be an increase in levels of fluorescence, due to increased PLA production. With only 1 MAGE cycles we were able generate a twofold increase, so in theory with an additional 5 MAGE cycles this increase will be even more drastic.
- This experiment will be done within the next week and the results will be presented at the Regional Competition
List of Papers:
Jacob et al. 1997
Matsuzaki et al. 1998
Sawers et al. 1998
Park et al. 2002
Selmer et al. 2002
Takase et al. 2002
Fong et al. 2005
Matsumoto et al. 2005
Rangarajan ES et al. 2005
Matsumoto et al. 2006
Jung et al. 2009
Matsumoto et al. 2009
Juang et al. 2010
Orth et al. 2010
Yang et al. 2011
Kandasamy et al. 2012
Yang et al. 2013
 "
"