Team:Imperial College/PHB production
From 2013.igem.org
Iain Bower (Talk | contribs) |
Iain Bower (Talk | contribs) |
||
| Line 8: | Line 8: | ||
O/N cultures of MG1655 transformed with either control or phaCAB plasmid were spread onto LB-agar plates with 3% glucose and Nile red staining. | O/N cultures of MG1655 transformed with either control or phaCAB plasmid were spread onto LB-agar plates with 3% glucose and Nile red staining. | ||
{| class="wikitable" style="margin: 1em auto 1em auto;" | {| class="wikitable" style="margin: 1em auto 1em auto;" | ||
| - | |[[File: | + | |[[File:EV_vs._phaCAB_red_-_reduced_background.PNG|thumbnail|right|400px|<b>Initial work with plastic synthesis in the native promoter.</b> Nile red staining was used to show expression of the plastic by fluorescence imaging. Control cells with empty vector are shown on the left, while native phaCAB transformed MG1655 is on the right.]] |
|[[File:27-9-13phaCABall.jpg|thumbnail|right|400px|<b>MG1655 constructs synthesising plastic.</b> Strains were grown on Nile red plates, which stain the PHB strongly and fluoresce in presence of PHB. On the left are MG1655 cells with an empty vector (no fluorescence; no plastic), at the bottom is the native promoter (i.e. low fluorescence, some plastic). At the top and right we have our constitutive and hybrid promoter (respectively), which both show high expression and thus fluoresce very clearly.]] | |[[File:27-9-13phaCABall.jpg|thumbnail|right|400px|<b>MG1655 constructs synthesising plastic.</b> Strains were grown on Nile red plates, which stain the PHB strongly and fluoresce in presence of PHB. On the left are MG1655 cells with an empty vector (no fluorescence; no plastic), at the bottom is the native promoter (i.e. low fluorescence, some plastic). At the top and right we have our constitutive and hybrid promoter (respectively), which both show high expression and thus fluoresce very clearly.]] | ||
|} | |} | ||
| Line 17: | Line 17: | ||
<h2 class="clear">Extraction of P3HB</h2> | <h2 class="clear">Extraction of P3HB</h2> | ||
| - | We extract P3HB using a technique which first disrupts the cell membranes and then degrades the remaining parts of the cell with bleach. See the [https://2013.igem.org/Team:Imperial_College/Protocols#P3HB_production_and_extraction protocols] section for more details. | + | <p>We extract P3HB using a technique which first disrupts the cell membranes and then degrades the remaining parts of the cell with bleach. See the [https://2013.igem.org/Team:Imperial_College/Protocols#P3HB_production_and_extraction protocols] section for more details.</p> |
| - | + | <p>Once the cultures have been centrifuged and the supernatant poured off , the biomass is clearly seen as cell pellets.</p> | |
| - | + | ||
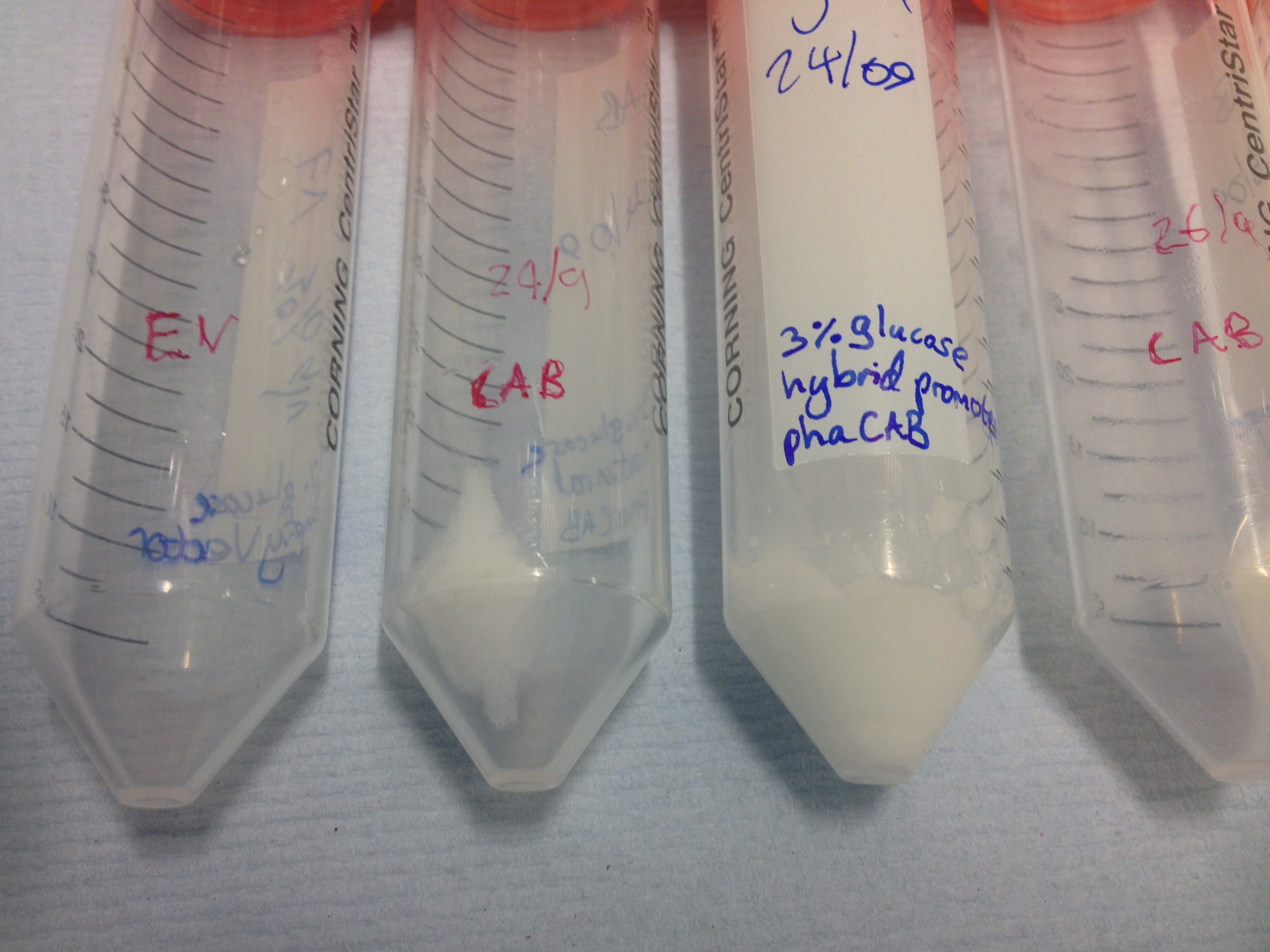
| - | File:Spun_down_hybrid_CAB_and_natural_CAB_3%25_glucose.jpg | + | {| class="wikitable" style="margin: 1em auto 1em auto;" |
| + | |[[File:Spun_down_EV_and_natural_phaCAB_grown_in_3%25_glucose.jpg|thumbnail|left|450px|Cell pellets left after the centrifugation of 300ml LB media with 3% glucose.]] | ||
| + | |[[File:Spun_down_hybrid_CAB_and_natural_CAB_3%25_glucose.jpg|thumbnail|right|450px|Cell pellets left after the centrifugation of 300ml LB media with 3% glucose. These pellets have slid back into the remaining supernatant.]] | ||
| + | |} | ||
{| class="wikitable" style="margin: 1em auto 1em auto;" | {| class="wikitable" style="margin: 1em auto 1em auto;" | ||
| - | |[[File:IMG_2191.JPG|thumbnail|left|200px|P(3HB) | + | |[[File:IMG_2191.JPG|thumbnail|left|200px|P(3HB) extracted from phaCAB transformed MG1655 that were grown in LB with 3% glucose]] |
| - | |[[File:IMG_2191.JPG|thumbnail|right|200px|P(3HB) | + | |[[File:IMG_2191.JPG|thumbnail|right|200px|P(3HB) extracted from phaCAB transformed MG1655 that were grown in LB with 3% glucose]] |
| - | |[[File:Plasticfrommixedwaste.jpg|thumbnail|center|250px|P(3HB) | + | |[[File:Plasticfrommixedwaste.jpg|thumbnail|center|250px|P(3HB) extracted from phaCAB transformed MG1655 that were grown in M9M mixed waste.]] |
|} | |} | ||
Revision as of 21:21, 29 September 2013
PHB production
During our project we successfully synthesised the bioplastic P(3HB).
Nile red staining
O/N cultures of MG1655 transformed with either control or phaCAB plasmid were spread onto LB-agar plates with 3% glucose and Nile red staining.
Conclusion: The red staining indicates the production of P(3HB). More importantly our new Biobricks [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1149051 hybrid promoter phaCAB BBa_K1149051] and [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1149052 constitutive phaCAB BBa_K1149052] produce more P(3HB) than the native phaCAB operon
Extraction of P3HB
We extract P3HB using a technique which first disrupts the cell membranes and then degrades the remaining parts of the cell with bleach. See the protocols section for more details.
Once the cultures have been centrifuged and the supernatant poured off , the biomass is clearly seen as cell pellets.
 "
"