Team:UNITN-Trento/Project/Methyl Salicylate
From 2013.igem.org
| Line 32: | Line 32: | ||
<div class= "tn-doublephoto-wrap"> | <div class= "tn-doublephoto-wrap"> | ||
| - | <img class="plot" style="height: 280px;" src="https://static.igem.org/mediawiki/2013/4/4f/Tn-2013_Taratura_MeSA.jpg"/> | + | <img class="plot" style="height: 280px; width: auto;" src="https://static.igem.org/mediawiki/2013/4/4f/Tn-2013_Taratura_MeSA.jpg"/> |
| - | <img class="plot" style="height: 280px;" src="https://static.igem.org/mediawiki/2013/7/79/Tn-2013_Pedro_GC.JPG" /> | + | <img class="plot" style="height: 280px; width: auto;" src="https://static.igem.org/mediawiki/2013/7/79/Tn-2013_Pedro_GC.JPG" /> |
</div> | </div> | ||
<span style="text-align:justify;" class="tn-caption center"><b>Figure 3:</b> Left panel: calibration curve obtained with different concentrations of pure MeSA in ethanol. We chose to use ethanol to build up the calibration curve because at the beginning we had some problems with the solubility of the methyl salicylate. However, during our experience were also performed many measures to verify the equivalence of using ethanol and LB as matrix for dissolving MeSA. Right panel: GC-FID in action </span> | <span style="text-align:justify;" class="tn-caption center"><b>Figure 3:</b> Left panel: calibration curve obtained with different concentrations of pure MeSA in ethanol. We chose to use ethanol to build up the calibration curve because at the beginning we had some problems with the solubility of the methyl salicylate. However, during our experience were also performed many measures to verify the equivalence of using ethanol and LB as matrix for dissolving MeSA. Right panel: GC-FID in action </span> | ||
Revision as of 13:04, 3 October 2013
B. fruity needed also a fruit ripening ihnibitor. It was difficult to find a volatile molecule that could be enzymatically produced by a bacteria and also proofed to be an efficient ripening inhbitor. There were not many candidates to choose from and after a long search we found methyl salicylate (MeSA). Previous work suggested that MeSA inhibits the ripening of kiwifruit (Aghdam M. et al., Journal of Agricultural Science. June 2011, Vol. 3, 2, pp. 149-156) and tomatoes, at a concentration of 0.5 mM (Ding, C. and Wang, Plant Science 2003, Y. 164 pp. 589-596).
We were happy to find out that many of the needed parts to produce MeSA were already available in the registry. These parts were initally built by the MIT 2006 iGEM team for the project Eau de coli.
 Figure 1: In this picture is shown the pathway that was exploited to produce Methyl Salicyalte. The precursor is the chorismate, a metabolic intermediate of the Shikimate pathway which many plants and bacteria (like E.coli and B.subtilis ) have. The chorismate undergoes firstly a reaction of isomerization by the isochorismate synthase, PchA and then the salicylate is obtained by the action of PchB an isochorismate pyruvate lyase. Both enzymes are from the micro-organism Pseudomonas aeruginosa . In the final part of the reaction, BSMT1, a methyltransferase, transfer a methyl group from the S-adenosyl-L-methionine synthesized by the SAM synthetase.
Figure 1: In this picture is shown the pathway that was exploited to produce Methyl Salicyalte. The precursor is the chorismate, a metabolic intermediate of the Shikimate pathway which many plants and bacteria (like E.coli and B.subtilis ) have. The chorismate undergoes firstly a reaction of isomerization by the isochorismate synthase, PchA and then the salicylate is obtained by the action of PchB an isochorismate pyruvate lyase. Both enzymes are from the micro-organism Pseudomonas aeruginosa . In the final part of the reaction, BSMT1, a methyltransferase, transfer a methyl group from the S-adenosyl-L-methionine synthesized by the SAM synthetase.
We modified and improved these parts and resubmitted them to the registry. For example, we substituted the pTet promoter controlling the BSMT1 enzyme with an araC-pBAD promoter. Additionally the MIT team did not include in their MeSA generator device the enzyme SAM synthetase, that we hope will boost MeSA production. We also have re-submitted in pSB1C3 the single enzymes of the pathway.
 MeSA detection
MeSA detection
MeSA is an highly volatile liquid with a distinct minty fragrance. We exploited the physical properties of MeSA to quantify its production by gas chromatography using a Finnigan Trace GC ULTRA connected to a flame ionization detector (FID). This kind of instrument, is able to detect ions formed during MeSA combustion in a hydrogen flame. The generation of this ions is proportional to MeSA concentration in the sample stream. A calibration curve was initially created using samples with a well known pure MeSA concentration (0 mM, 0.2 mM, 0.5 mM, 1.0 mM, 2 mM). For more details about the protocol that we used for the instrument see here


 Figure 4: induced sample produces MeSA. A culture of cells transformed with BBa_K1065102 was grown until O.D. 0.6 was reached. The culture was then splitted in 2 samples and one was induced with 5 mM arabinose. 2 mM salycilic acid was added to these samples. After about 4 h the samples were connected to the Gas Chromatograph. The induced sample (blue trace) shows the characteristic peak of methyl salicylate, as opposed to non induced cells (red trace).
Once we had all the chromatograms, with the software Finningan Xcalibur® , we were able to obtain directly the MeSA quantities from each bacteria’s samples. Below we have reported the most significant data.
Figure 4: induced sample produces MeSA. A culture of cells transformed with BBa_K1065102 was grown until O.D. 0.6 was reached. The culture was then splitted in 2 samples and one was induced with 5 mM arabinose. 2 mM salycilic acid was added to these samples. After about 4 h the samples were connected to the Gas Chromatograph. The induced sample (blue trace) shows the characteristic peak of methyl salicylate, as opposed to non induced cells (red trace).
Once we had all the chromatograms, with the software Finningan Xcalibur® , we were able to obtain directly the MeSA quantities from each bacteria’s samples. Below we have reported the most significant data.
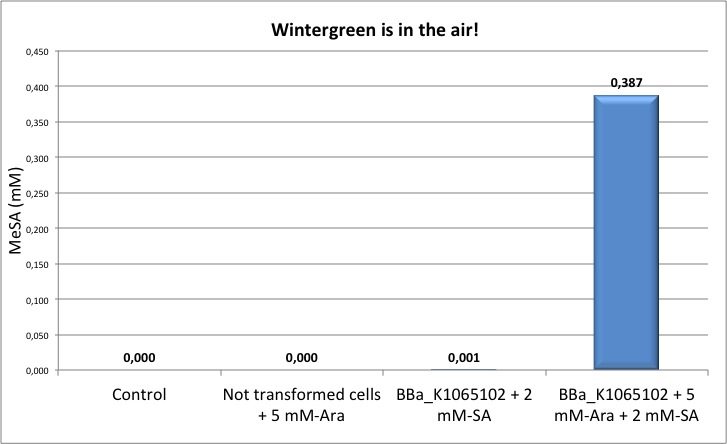 Figure 5: Quantification of MeSA by GC-FID. NEB10β cells transformed with BBa_K1065102 supplemented with salycilic acid produce around 0.4 mM of MeSA. Non transformed cells and non induced cells did not produce any MeSA. Cells induced with arabinose and not supplemented with salycilic acid did not show any significant MeSA concentration (data not shown). MANCA GRAFICO
Our MeSA device BBa_K1065102 was able to produce a significant concentration of MeSA only in the presence of salycilic acid. This finding was also previously observed by the MIT team in 2006 with their device (BBa_J45700). This result was initially imputed to the lack of SAM synthetase. However, after we received the DNA sequencing results of the MIT part (BBa_J45300) and of our complete device (built with MIT parts) we realised that the pLAC promoter was missing the -35 box, thus generating a less strong promoter. We believe that this problem can significantly affect the correct functioning of the device. We are now in the process of improving this part by mutagenesis to rebuild a full functional pLAC promoter.
For the details about how we employed MeSA to block the fruit maturation visit the Fruit Ripening page.
Figure 5: Quantification of MeSA by GC-FID. NEB10β cells transformed with BBa_K1065102 supplemented with salycilic acid produce around 0.4 mM of MeSA. Non transformed cells and non induced cells did not produce any MeSA. Cells induced with arabinose and not supplemented with salycilic acid did not show any significant MeSA concentration (data not shown). MANCA GRAFICO
Our MeSA device BBa_K1065102 was able to produce a significant concentration of MeSA only in the presence of salycilic acid. This finding was also previously observed by the MIT team in 2006 with their device (BBa_J45700). This result was initially imputed to the lack of SAM synthetase. However, after we received the DNA sequencing results of the MIT part (BBa_J45300) and of our complete device (built with MIT parts) we realised that the pLAC promoter was missing the -35 box, thus generating a less strong promoter. We believe that this problem can significantly affect the correct functioning of the device. We are now in the process of improving this part by mutagenesis to rebuild a full functional pLAC promoter.
For the details about how we employed MeSA to block the fruit maturation visit the Fruit Ripening page.
 "
"
















