Team:Newcastle/Project
From 2013.igem.org
YDemyanenko (Talk | contribs) |
m |
||
| Line 22: | Line 22: | ||
====[https://2013.igem.org/Team:Newcastle/Project/shuffling_endosymbiosis Genome Shuffling]==== | ====[https://2013.igem.org/Team:Newcastle/Project/shuffling_endosymbiosis Genome Shuffling]==== | ||
| - | Fusion of bacteria is made significantly easier without a cell wall acting as a sheer physical obstacle. Cell fusion forces the fusants to reproduce sexually, where their genomes recombine. This can be used in genome shuffling, whereby genetic material is exchanged between the genomes of the fused cells. Furthermore the process can be utilised | + | Fusion of bacteria is made significantly easier without a cell wall acting as a sheer physical obstacle. Cell fusion forces the fusants to reproduce sexually, where their genomes recombine. This can be used in genome shuffling, whereby genetic material is exchanged between the genomes of the fused cells. Furthermore the process can be utilised in directed evolution, whereby certain characteristics that may be altered by this shuffling of genetic material are selected for. |
====[https://2013.igem.org/Team:Newcastle/Project/plants Introduction and Detection of Naked Bacteria in Plants]==== | ====[https://2013.igem.org/Team:Newcastle/Project/plants Introduction and Detection of Naked Bacteria in Plants]==== | ||
Revision as of 19:56, 4 October 2013

Contents |
Project Overview
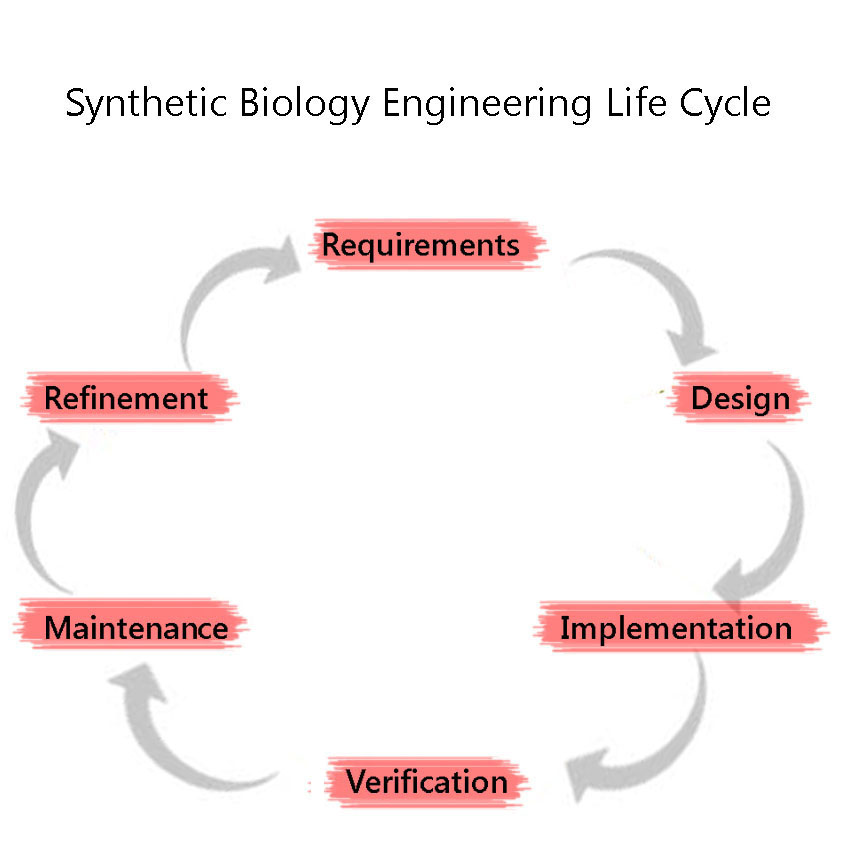
Synthetic Biology is a discipline which heavily employs engineering principles. One of these principles is the Engineering Lifecycle, a framework in which the project is split into clearly defined sections, based on the development of the project. These are, in sequential order: 1) Requirements 2) Design (including Modelling) 3) Implementation 4) Verification 5) Maintenance 6)Refinement and the cycle is iterated through again, as we attempt to improve the system further. We adhered to this cycle throughout our project including the development and characterisation of our BioBricks.
A Foundational Advance
While researching Synthetic Biology we found that a cell wall, one of the standard components of the bacterial cell, often causes difficulties in many techniques. These included transformation efficiency, secretion of recombinant proteins, adaption to the environment etc. So we thought to ourselves: is there any way to remove the cell wall and still have a viable cell?
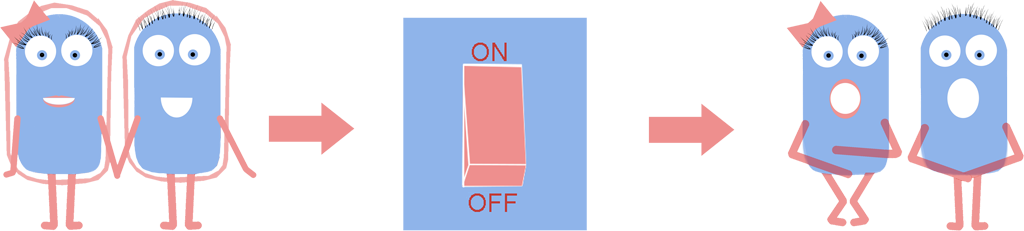
As a result we have developed a new chassis with the potential to revolutionise how Synthetic Biology is performed. The main BioBrick(BBa_K1185000) that we have introduced enables the switching on and off of the bacterial cell wall in the model Gram positive bacteria Bacillus subtilis, at the demand of the synthetic biologist, while still allowing cells to grow and divide. Employing bacterial cells without a cell wall can both enable the synthetic biologist to explore new applications and research areas, and also build-upon and improve areas that are already being explored in Synthetic Biology. Rather than the application-oriented nature of many iGEM projects, we think that the use of cell wall-less bacteria as a novel chassis in Synthetic Biology, as we propose, can benefit across the whole subject area, and furthermore be utilised as a tool to allow for even greater feats to be achieved by future iGEM teams.
Bacteria which have lost their cell wall yet are still able to grow and divide are called L-forms, or as we prefer to call them, naked bacteria. If you would like to learn more about L-forms, please take a look at our L-form page. Once we had created L-forms with our Switch BioBrick, we were unable to ignore the new opportunities that L-forms bring to Synthetic Biology:
Click [http://www.youtube.com/watch?v=b0Kk6bKKOQ0 here] to watch a video summarising our project.
Genome Shuffling
Fusion of bacteria is made significantly easier without a cell wall acting as a sheer physical obstacle. Cell fusion forces the fusants to reproduce sexually, where their genomes recombine. This can be used in genome shuffling, whereby genetic material is exchanged between the genomes of the fused cells. Furthermore the process can be utilised in directed evolution, whereby certain characteristics that may be altered by this shuffling of genetic material are selected for.
Introduction and Detection of Naked Bacteria in Plants
L-forms have been shown to form symbiosis in plants. Plants with naked bacteria show increased resistance to fungus and they could be used to deliver useful compounds to the plant. This could give better crop yields, more nutritious harvests and reduce the need for spraying of fertiliser, pesticides or other compounds.
Shape Shifting
The loss of the cell wall leaves L-forms protected by only a cell membrane. The plasma membrane of L-forms is quite fluid. The advantage of this is that these cells would be able to adapt to shapes of various cracks and cavities, or will be able to "squeeze through" tiny channels and deliver cargo to hard-to reach targets.
This isn’t a finite list of what can be done with naked bacteria, there’s loads more! L-forms are currently used to discover novel antibiotics which don’t act on the cell wall. L-forms can also teach us a great deal about how bacterial life has evolved, through acting as a model for a cell wall-less bacterial progenitor, and through being able to test the ease of induction of endosymbiosis in cell wall-less organisms (Mercier et al. 2013).
L-form bacteria can be used in any process which protoplasts are currently used for. Protoplasts are bacteria which have been chemically induced to lose their cell wall. They cannot however grow or divide (as L-forms can) and are not classified as being alive. L-forms can be used to transform bacteria which are recalcitrant to transformation (Chang and Cohen 1979).
One crucially important feature of our naked bacteria is that they are osmotically sensitive, meaning that they will lyse if they escape into the environment. This means that they can be used in non-contained environments (e.g. agriculture).
Requirements
Each of our chosen research themes had a set of specific requirements, i.e. BioBricks, equipment and, of course, a research strategy. However, all aspects of our research relied upon the use of L-forms (naked bacteria), specifically bacteria that could switch between walled and wall-less states. It was crucial that we devised a BioBrick that, once integrated allowed for the switching of rod cells to L-form (and back again).
Genome Shuffling
To explore the possibilities of using L-forms for directed evolution, we have designed two BioBricks to differentially tag the DNA of different cells with fluorescent proteins. This has allowed us to visually observe fusion events and to be able to reliably identify genetic recombination between the genomes of fused cells. The BioBricks were designed without a promoter, so they could be cloned into any plasmid under the control of the desired promoter and be used in various bacterial species.
For our research we used pMutin4 plasmids. However before deciding to use this particular plamid we have tested a plasmid designed by the Groningen 2012 iGEM team. We were unable to use the plasmid and we have thus managed to [http://parts.igem.org/Part:BBa_K818000:Experience characterise it] and speculate why this part is non-functional.
Introduction and Detection of Naked Bacteria in Plants
To find out whether L-forms could happily live among plant rot cells we have cultivated them in a variety of plants including chinese cabbage and strawberries. To visualise the L-forms inside the roots we have used a GFP protein reporter gene, and observed the cells under a confocal microscope.
Shape Shifting
The loss of the cell wall leaves L-forms protected by only a cell membrane. The plasma membrane of l-forms is quite fluid. The advantage of this is that these cells would be able to adapt to shapes of various cracks and cavities, or will be able to "squeeze through" tiny channels and deliver cargo to hard-to reach targets.
We have planned to test this hypothesis by injecting the naked bacteria into specially designed microfluidics chambers and observing their behaviour under the microscope. Membrane would have been visualised with a commonly used membrane stain F.595
Modelling
The next step on the diagram is modelling. This step is an essential part of a successful synthetic biology project. Although it requires a lot of time and effort, and therefore is often neglected. We believe that in the long run modelling can save a lot of time, effort and resources to those who take their time in the beginning, simulating all the possible outcomes of the system and refining it at an early stage, before any in vitro and in vivo experiments have been planned and conducted. Another positive side to modelling prior to the "wet lab" sessions is the fact that a model behaves according to the known facts and principles, and if in real life the outcome drastically differs from the simulation, there's a good chance of finding out what may be causing the difference through adjusting the model and repeating the experiments.
For every research theme we have constructed a model to help us understand the systems we engineered. Click on the links to view each model or visit our modelling page:
Implementation
What we've done for each project (brief summary). Assuming the same format as Analysis.
Verification
By the end of our project we have:
- created and characterised the BioBrick which allows the switching on and off of the cell wall of ‘’B.subtilis”.
- Shown that the naked bacteria that we created using our switch BioBrick also form these associations.
All you need to start using an L-form chassis is a culture of Bacillus subtilis, our L-form switch BioBrick and a set of instructions from us.
References
[http://www.ncbi.nlm.nih.gov/pubmed/11849491 Walker R, Ferguson CMJ, Booth NA and Allan EJ (2002) The symbiosis of Bacillus subtilis L-forms with Chinese cabbage seedlings inhibits conidial germination of ‘Botrytis cinerea. Letters in Applied Microbiology, 34, 42-45.]
[http://www.ncbi.nlm.nih.gov/pubmed/107388 Chang S and Cohen SN (1979)High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Molecular Genetics & Genomics, 168, 111–115.]
[http://www.cell.com/abstract/S0092-8674(13)00135-9 Mercier R, Kawai Y and Errington J. (2013) Excess Membrane Synthesis Drives a Primitive Mode of Cell Proliferation, Cell, 152, 997–1007.]
 "
"