Team:Paris Bettencourt/Project/Target
From 2013.igem.org
| Line 65: | Line 65: | ||
</div> | </div> | ||
<div class="rightparagraph"> | <div class="rightparagraph"> | ||
| - | <img src="https://static.igem.org/mediawiki/2013/4/4f/PS_Drug_Scheme.png" width="535px"/> | + | <a href="https://static.igem.org/mediawiki/2013/4/4f/PS_Drug_Scheme.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/4/4f/PS_Drug_Scheme.png" width="535px"/></a> |
<p><b>Figure 1: Overview of Targeted Drug Screen Design</b></p> | <p><b>Figure 1: Overview of Targeted Drug Screen Design</b></p> | ||
</div> | </div> | ||
| Line 77: | Line 77: | ||
</div> | </div> | ||
<div class="rightparagraph"> | <div class="rightparagraph"> | ||
| - | <center><img src="https://static.igem.org/mediawiki/2013/7/76/PS_FBA.png" width="267.5px"/></center> | + | <center><a href="https://static.igem.org/mediawiki/2013/7/76/PS_FBA.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/7/76/PS_FBA.png" width="267.5px"/></a></center> |
<p><b>Figure 2: Biomass Flux through E. coli and mycoSIR E. coli</b><div style="font-size: 90%">Flux balance analysis was run using Cobra Toolbox 2.0 on E. coli sbml model iJR904 with and without SULR reaction. Additionally an E. coli sbml model was built with the SULR reaction replaced with a reaction representing the mycobacterial SirA reaction and FprA reaction, as well as ferredoxin FdxA as an additional species. The Biomass flux is restored to 99.75% of the wild-type level with the synthetic mycobacterial system.</div></p> | <p><b>Figure 2: Biomass Flux through E. coli and mycoSIR E. coli</b><div style="font-size: 90%">Flux balance analysis was run using Cobra Toolbox 2.0 on E. coli sbml model iJR904 with and without SULR reaction. Additionally an E. coli sbml model was built with the SULR reaction replaced with a reaction representing the mycobacterial SirA reaction and FprA reaction, as well as ferredoxin FdxA as an additional species. The Biomass flux is restored to 99.75% of the wild-type level with the synthetic mycobacterial system.</div></p> | ||
</div> | </div> | ||
| Line 104: | Line 104: | ||
</div> | </div> | ||
<div class="rightparagraph"> | <div class="rightparagraph"> | ||
| - | <center><img src="https://static.igem.org/mediawiki/2013/5/59/Drug_target_withoutAA.png" width="267.5px"/></center> | + | <center><a href="https://static.igem.org/mediawiki/2013/5/59/Drug_target_withoutAA.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/5/59/Drug_target_withoutAA.png" width="267.5px"/></a></center> |
<p><b>Figure 4 Drug target locations in SirA </b><div style="font-size: 90%">A domain located in SirA, identified as a drug target through Chembl analysis.</div></p> | <p><b>Figure 4 Drug target locations in SirA </b><div style="font-size: 90%">A domain located in SirA, identified as a drug target through Chembl analysis.</div></p> | ||
</div> | </div> | ||
| Line 118: | Line 118: | ||
</div> | </div> | ||
<div class="rightparagraph"> | <div class="rightparagraph"> | ||
| - | <img src="https://static.igem.org/mediawiki/2013/8/84/TB_drug_FinalSmeg.png" width="535px"/> | + | <a href="https://static.igem.org/mediawiki/2013/8/84/TB_drug_FinalSmeg.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/8/84/TB_drug_FinalSmeg.png" width="535px"/></a> |
<p><b>Figure 5: Growth curves of E coli mycoSIR</b> <div style="font-size: 90%">BL21 (DE3) ΔcysI containing the MycoSIR pathway (MycoSIR E.coli) were grown in liquid minimal media containing Various concentration of IPTG. (A) Replicates of each strain were measured for absorbance in a spectrophotometer every 10 minutes for 14 hours. Growth was observed for the WT BL21 E.coli, (blue), and the MycoSIR E.coli (red). No growth was detected for uninduced MycoSIR E.coli (purple) or for the BL21 (DE3) ΔcysI that did not contain the synthetic pathway (Orange) . (B) Mean Final ODs of all replicates, measured after 14 hours of growth. Growth was detected in zmSIR E.coli and WT BL21 but not in uninduced zmSIR strain.</div></p> | <p><b>Figure 5: Growth curves of E coli mycoSIR</b> <div style="font-size: 90%">BL21 (DE3) ΔcysI containing the MycoSIR pathway (MycoSIR E.coli) were grown in liquid minimal media containing Various concentration of IPTG. (A) Replicates of each strain were measured for absorbance in a spectrophotometer every 10 minutes for 14 hours. Growth was observed for the WT BL21 E.coli, (blue), and the MycoSIR E.coli (red). No growth was detected for uninduced MycoSIR E.coli (purple) or for the BL21 (DE3) ΔcysI that did not contain the synthetic pathway (Orange) . (B) Mean Final ODs of all replicates, measured after 14 hours of growth. Growth was detected in zmSIR E.coli and WT BL21 but not in uninduced zmSIR strain.</div></p> | ||
</div> | </div> | ||
| Line 137: | Line 137: | ||
</div> | </div> | ||
<div class="rightparagraph"> | <div class="rightparagraph"> | ||
| - | <img src="https://static.igem.org/mediawiki/2013/a/a2/PB_final_Corn.png" width="535px"/> | + | <a href="https://static.igem.org/mediawiki/2013/a/a2/PB_final_Corn.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/a/a2/PB_final_Corn.png" width="535px"/></a> |
<p><b>Figure 6: Growth curves of E coli maizeSIR</b><div style="font-size: 90%"> BL21 (DE3) ΔcysI containing the MaizeSIR pathway (MaizeSIR E.coli) were grown in liquid minimal media containing Various concentration of IPTG. (A) Replicates of each strain were measured for absorbance in a spectrophotometer every 10 minutes for 14 hours. Growth was observed for the WT BL21 E.coli, (blue), and the MaizeSIR E.coli (red). No growth was detected for uninduced MaizeSIR E.coli (purple) or for the BL21 (DE3) ΔcysI that did not contain the synthetic pathway (Orange) . (B) Mean Final ODs of all replicates, measured after 14 hours of growth. Growth was detected in zmSIR E.coli and WT BL21 but not in uninduced zmSIR strain.</div></p> | <p><b>Figure 6: Growth curves of E coli maizeSIR</b><div style="font-size: 90%"> BL21 (DE3) ΔcysI containing the MaizeSIR pathway (MaizeSIR E.coli) were grown in liquid minimal media containing Various concentration of IPTG. (A) Replicates of each strain were measured for absorbance in a spectrophotometer every 10 minutes for 14 hours. Growth was observed for the WT BL21 E.coli, (blue), and the MaizeSIR E.coli (red). No growth was detected for uninduced MaizeSIR E.coli (purple) or for the BL21 (DE3) ΔcysI that did not contain the synthetic pathway (Orange) . (B) Mean Final ODs of all replicates, measured after 14 hours of growth. Growth was detected in zmSIR E.coli and WT BL21 but not in uninduced zmSIR strain.</div></p> | ||
</div> | </div> | ||
| Line 149: | Line 149: | ||
</div> | </div> | ||
<div class="rightparagraph"> | <div class="rightparagraph"> | ||
| - | <img src="https://static.igem.org/mediawiki/2013/8/85/PS_D_Figure1_plate.png" width="535px"/> | + | <a href="https://static.igem.org/mediawiki/2013/8/85/PS_D_Figure1_plate.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/8/85/PS_D_Figure1_plate.png" width="535px"/></a> |
<p><b>Figure 7: Growth of zmSIR E.coli on minimal media.</b> <div style="font-size: 90%">BL21 (DE3) ΔcysI cells transformed with 1, 2 and 3 genes of the 3-gene zmSIR synthetic pathway were grown for 24 hours on minimal media supplemented with 25 uM IPTG (see methods), along with a WT BL21 (DE3) serving as a negative control, and an untransformed BL21 (DE3) ΔcysI, as negative control. Rescue of growth required all genes of the synthetic pathway (SIR, FNR and FD). </div></p> | <p><b>Figure 7: Growth of zmSIR E.coli on minimal media.</b> <div style="font-size: 90%">BL21 (DE3) ΔcysI cells transformed with 1, 2 and 3 genes of the 3-gene zmSIR synthetic pathway were grown for 24 hours on minimal media supplemented with 25 uM IPTG (see methods), along with a WT BL21 (DE3) serving as a negative control, and an untransformed BL21 (DE3) ΔcysI, as negative control. Rescue of growth required all genes of the synthetic pathway (SIR, FNR and FD). </div></p> | ||
</div> | </div> | ||
Revision as of 23:53, 26 October 2013


Background
SirA is an essential gene in latent tuberculosis infections
Results
- Produced an E. coli strain which relies upon mycobacterial sirA, fprA and fdxA genes to survive in M9 minimal media
- Demonstrated that E. coli can survive with mycobacterial sulfite reduction pathway with Flux Balance Analysis
- Located drug target sites on sirA as well as identified high structural similarity between cysI and sirA through structural anaylsis
Aims
To perform a drug screen targeted at the sirA gene from mycobacteria
Skip to Introduction
Skip to Modeling
Skip to Design
Skip to Results
Introduction
SirA is essential for M. tuberculosis persistence phenotype as sulfur containing amino acids are particularly sensitive to oxidative stress within the macrophage and must regularly be replaced. Currently, there are no drug candidates that are known to specifically inhibit SirA and conventional drug screens involve do not provide information regarding the mechanism of drug action nor do compounds that inhibit exponential growth necessarily have an effect on persistent TB. We designed a working drug screen assay to specifically target the mycobacterial sulfite reductase protein SirA. To this end we cloned Ito E. coli the sulfite reduction pathway of M.Smegmatis, a non-pathogenic mycobacterial relative of M. Tuberculosis. Our model overcomes the problem of long doubling time of M. tuberculosis. Specific inhibition of the sulfite reduction pathway is scored by comparing a drug screen of our E. coli construct vs. wild-type. Any drug candidates that have activity against both the wild-type E. coli and our construct are non-specific inhibitors of E. coli growth. However, any drug candidates that inhibit only the growth of our E. coli construct will be SirA pathway specific.
Flux Balance Analysis of Sulfite Reduction Pathway
We used an E. coli model (iJR904) obtained from the BiGG database as a starting model to obtain wild-type growth rate (f = 0.9129). We then deleted the reaction ‘SULR’ which encodes for the sulphite reduction pathway involving cysI and obtained a f= -8e-13=0 indicating that the sulphite reduction pathway is essential for growth. Finally we introduced two new reactions for sirA and fprA and a new species fdxA. We found that growth with the mycobacteria pathway reverts the growth phenotype back to wild-type levels (f = 0.9105).
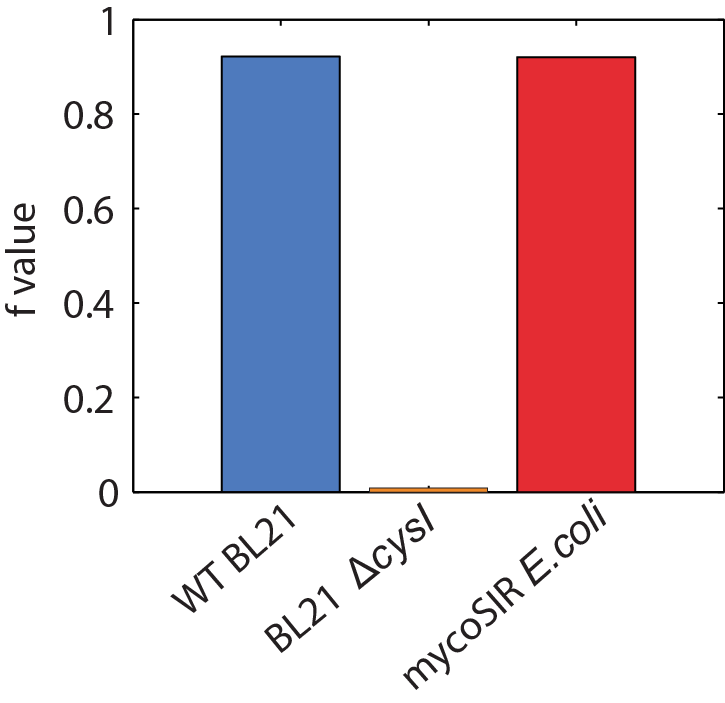
Figure 2: Biomass Flux through E. coli and mycoSIR E. coli
Structural Analysis of SirA
Superimposing the structures of M.tuberculosis SirA and E.coli CysI reveals high homology, in particular of the active sites. Both proteins have the same symmetry (psuedo 2 fold) indicative of a common evolutionary origin. Our analysis highlighted important conserved residues, involved in substrate binding to be Arg97, Arg130, Arg166, Lys207. These positively charged residues are conserved in the sulphite/nitrite reductase family. In addition, 4 Cys residues are conserved for iron-sulphur binding.
The most profound structural differences between the two enzymes are found in the ferredoxin binding site and SirA's most C terminal residues and several surface loop regions due to deletions or insertions. A stark difference is a covalent bond formed between Cys161 (thiolate) and Tyr69 (C carbon atom) found adjacent to the redox center (Cu ions) in SirA. The covalently bound residues act as a secondary cofactor in tyrosyl radical stabilization.

Figure 3: The superimposed 3D protein structures of SirA and CysI.
Identification of potential drug target binding sites
Our structural analysis provided the basis for our drug target prediction. Using Chembl and swiss pdb, we have shown a predicted drug target site. Our calculation gives strong favour for a drug to be effective at this site. The calculation reflects the suitability of small molecules to the binding site under the Lipinski's Rule of 5.
The drug target is located at the interface of the three domains. This binding pocket exhibits a dense hydrophobic region. Our analysis targets 48 amino acids of SirA within 6Å of a modelled small drug molecule. Of these residues, only 6 amino acids are charged: His409, Asp453, Asp474, His500, Asp504 and Arg541.
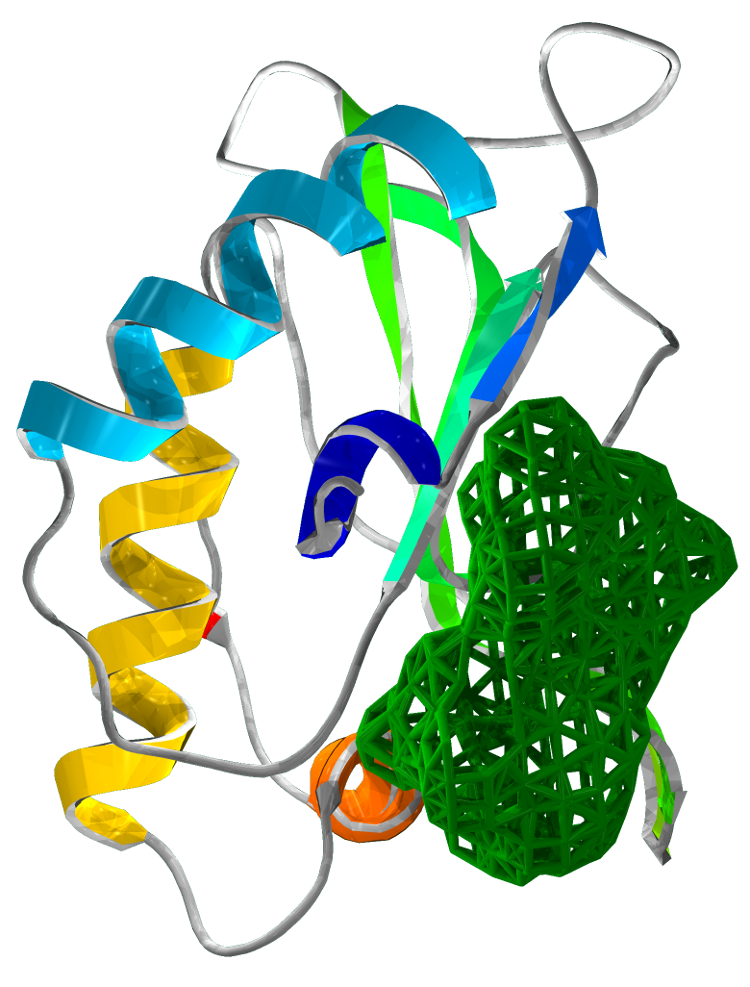
Figure 4 Drug target locations in SirA
Synthetic Mycobacteria Pathway
We designed a synthetic M.smegmatis-derived sulfite reduction pathway containing sirA - the sulfite reductase, and two supporting genes that are required for its function in E.coli: fdxA and fprA. FdxA is a mycobacterial Ferredoxin cofactor which is oxidised by SirA during the sulfite reduction reaction and FprA is a Ferredoxin-NADPH reductase use replenish the reduced Fdx pool. The genes' sequences were taken from previous work describing their expression in E.coli for purification and in vitro characterization; we removed restriction sites and codon optimized for expression in E. coli. The genes were then cloned into two Duet expression vectors, one containing sirA and one containing the supporting genesand were transformed into our knock-out mutant strains of E. coli. Data on Growth curves can be found at https://2013.igem.org/Team:Paris_Bettencourt/ Notebook/Drug_Screening/Monday_30th_September.html

Figure 5: Growth curves of E coli mycoSIR
Creation of Knock out Mutants
We prepared two strains of E. coli which have the sulfite reduction pathway deleted: BL21 (DE3) ΔCysI Δfpr ΔydbK and BL21 (AI) ΔCysI. CysI is responsible for sulfite reduction in E. coli, while fpr and ydbK are two non-essential genes that consume ferredoxin. These two genes are deleted, as sulfite reduction in mycobacteria is ferredoxin dependent in comparison to E. coli in which it is NADPH dependant. To ensure that these two genes do not interfere with our system, we deleted these genes as well.
Synthetic Corn Pathway
Additionally we prototyped the system with a reconstruction of a sulphite reduction pathway previously designed and published by the silver group (2011 Barstow et al). In place of CysI, a corn (Zea mays) derived sulfite reductase (zmSIR) was used. Two additional genes were included: Spinach ferredoxin (soFD), and corn derived ferredoxin NADP+ reductase (zmFNR). These genes, respectively, are required for production of the ferredoxin cofactor and the NADP+ ferredoxin reductase and are required for sulfite reductase (zmSIR) to function within E. coli.

Figure 6: Growth curves of E coli maizeSIR
Results
Upon successful cloning of the three genes into our E. coli deletion strain, we continued to confirm that all three genes are required for growth on minimal media. Our two synthetic pathways were found to rescue growth on a sulfurless amino acid supplemented minimal media. We hope that this technique of using synthetic biology to overcome problems faced in naturally occurring systems will be both a large boon to the pursuit of finding novel drug candidates in M. tuberculosis and more broadly as this technique can be used for high-throughput screening of any pathway that can be constructed to be essential for growth in E. coli.

Figure 7: Growth of zmSIR E.coli on minimal media.
 "
"








 +33 1 44 41 25 22/25
+33 1 44 41 25 22/25


