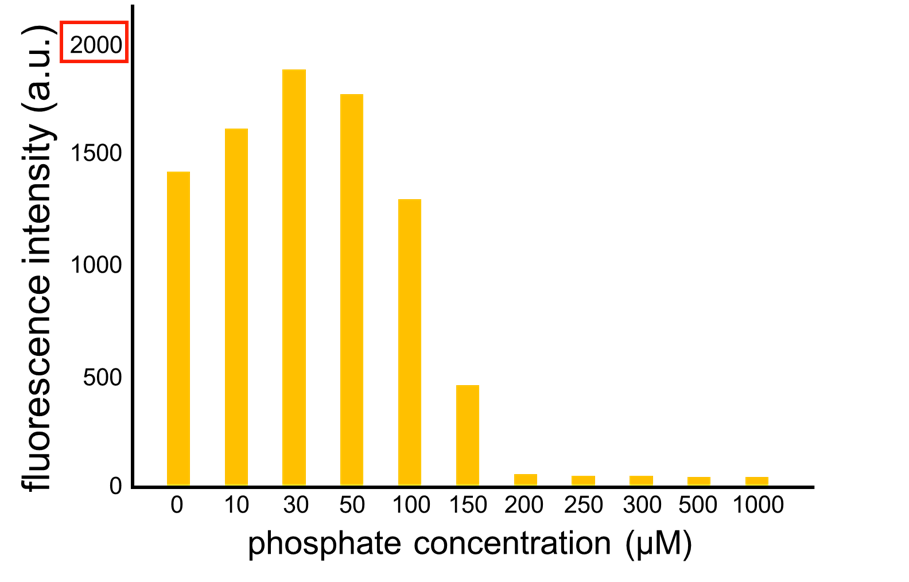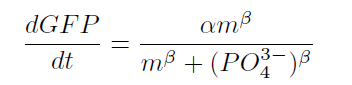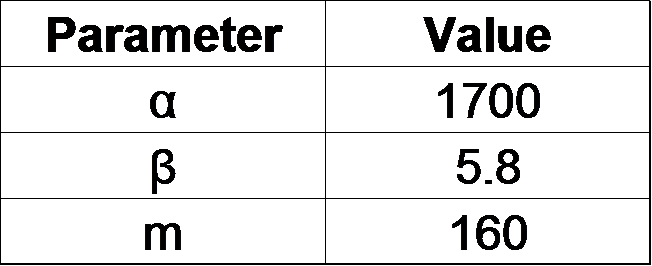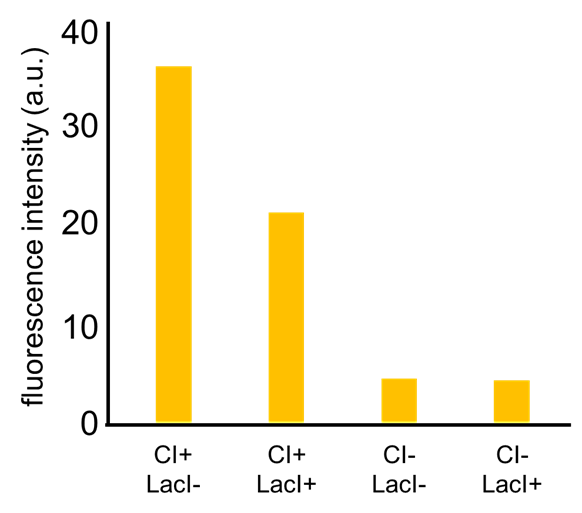Team:Tokyo Tech/Project/Farming
From 2013.igem.org
| Line 62: | Line 62: | ||
<h1>3. Cytokinin synthesis</h1><h2> | <h1>3. Cytokinin synthesis</h1><h2> | ||
<p> | <p> | ||
| - | In order to construct cytokinin production part, we focused on AtIPT, a plant enzyme which catalyzes the synthesis of cytokinin. The mechanisms of cytokinin synthesis are shown in Fig. 2-3-10 (Takei et al., 2001). We ordered two DNA sequences of <i>At</i>IPT (<i>At</i>IPT4 and <i>At</i>IPT7) derived from A. thaliana and | + | In order to construct cytokinin production part, we focused on AtIPT, a plant enzyme which catalyzes the synthesis of cytokinin. The mechanisms of cytokinin synthesis are shown in Fig. 2-3-10 (Takei et al., 2001). We ordered two DNA sequences of <i>At</i>IPT (<i>At</i>IPT4 and <i>At</i>IPT7) derived from <i>A. thaliana</i> and constructed the part including one of them. |
</p> | </p> | ||
[[Image:Titech2013_farming_Fig_2-3-10.png|600px|thumb|center|Fig. 2-3-10. Mechanisms of cytokinin synthesis]] | [[Image:Titech2013_farming_Fig_2-3-10.png|600px|thumb|center|Fig. 2-3-10. Mechanisms of cytokinin synthesis]] | ||
| Line 68: | Line 68: | ||
<p> | <p> | ||
<div style="margin-left:80px;"> | <div style="margin-left:80px;"> | ||
| - | AtIPT, a plant enzyme, catalyzes the synthesis of iPRMP from DMAPP and AMP. According to the previous research, DMAPP, and AMP are provided by authentic metabolism in <i>E. coli</i> . It is also expected in the report that <i>E. coli</i> also has the enzymes which catalyze the synthesis of iP or tZ from iPRMP. | + | AtIPT, a plant enzyme, catalyzes the synthesis of iPRMP from DMAPP and AMP. According to the previous research, DMAPP, and AMP are provided by authentic metabolism in <i>E. coli</i> . It is also expected in the report that <i>E. coli</i> also has the enzymes which catalyze the synthesis of iP or tZ (both sorts of cytokinin) from iPRMP. |
</div></p></h5> | </div></p></h5> | ||
</div><br> | </div><br> | ||
| Line 74: | Line 74: | ||
<h1>4. Quantitative analysis for cytokinin by bioassay</h1><h2> | <h1>4. Quantitative analysis for cytokinin by bioassay</h1><h2> | ||
<p> | <p> | ||
| - | Before constructing genetic parts for cytokinin synthesis, we learned methods for quantitative analysis for cytokinin through a bioassay of cucumber seed sprouts ( | + | Before constructing genetic parts for cytokinin synthesis, we learned methods for quantitative analysis for cytokinin through a bioassay of cucumber seed sprouts (Fletcher et al., 1971; Porra et al., 1989) and through using ultra-performance liquid chromatography (UPLC). |
</p> | </p> | ||
<p> | <p> | ||
| - | In our bioassay of cucumber seed sprouts, we | + | In our bioassay of cucumber seed sprouts, we learned how to detect cytokinin and found which concentration of cytokinin can act on plants. We planted the seeds and germinated for 5 days. Then, we cultivated the sprouts in standard cytokinin sample solutions. After 24 hours in the dark and 24 hours in the light, we measured the weight of the sprouts and also homogenized them to measure the concentration of chlorophyll (Fig. 2-3-12). Fig. 2-3-11 shows the result that the sprouts in cytokinin solution are larger and greener than the negative controls, which are the sprouts in solution without cytokinin. The quantitative results (Fig. 2-3-13 and Fig. 2-3-14) also show that cytokinin increases the weight of the sprouts and the concentration of chlorophyll. To know more about this assay, please see <font size ="5">[https://2013.igem.org/Team:Tokyo_Tech/Experiment/Quantitative_Analysis_of_Cytokinin#1._Quantitative_analysis_of_cytokinins_using_cucumber_cotyledons here]</font size>. |
</p> | </p> | ||
<gallery widths="350px" heights="160px" style="margin-left:auto; margin-right:auto;"> | <gallery widths="350px" heights="160px" style="margin-left:auto; margin-right:auto;"> | ||
| Line 91: | Line 91: | ||
<div style="margin-left:80px;"> | <div style="margin-left:80px;"> | ||
iP : 6-(γ, γ-Dimethylallylamino) purine, tZ : trans-zeatin<br> | iP : 6-(γ, γ-Dimethylallylamino) purine, tZ : trans-zeatin<br> | ||
| - | The | + | The absorbances of the supernatant were read at 663.6 and 646.6 nm. Calculation of concentrations of the chlorophyll were carried out as described by Porra et al.,1989. |
</div></p> | </div></p> | ||
</h5><h2> | </h5><h2> | ||
<p> | <p> | ||
| - | Through ultra-performance liquid chromatography (UPLC), we | + | Through ultra-performance liquid chromatography (UPLC), we planned to confirm that our "Farmer <i>E. coli</i>" actually produce cytokinin. Before completing the system to produce cytokinin in <i>E. coli</i>, we determined the retention times of authentic samples of iP and tZ by using UPLC. Fig. 2-3-15 shows the result. In addition, we confirmed that iP and tZ were able to be detected from the mixture of the <i>E. coli</i> culture medium and the cytokinin solution. To see the detail method of this experiment, please click [https://2013.igem.org/Team:Tokyo_Tech/Experiment/Quantitative_Analysis_of_Cytokinin#2._Identification_of_cytokinins_by_ultra-performance_liquid_chromatography_.28UPLC.29 <font size ="5">here</font size>]. |
</p> | </p> | ||
| - | [[Image:Titech2013_farming_Fig_2-3-15.png|700px|thumb|center|Fig. 2-3-15. The | + | [[Image:Titech2013_farming_Fig_2-3-15.png|700px|thumb|center|Fig. 2-3-15. The retension times of iP and tZ standards determined by UPLC]] |
</h2></div><br> | </h2></div><br> | ||
<div class="box"> | <div class="box"> | ||
<h1>5. Hybrid promoter for temporal pattern generation</h1><h2> | <h1>5. Hybrid promoter for temporal pattern generation</h1><h2> | ||
<p> | <p> | ||
| - | In order to achieve the natural plants’ temporal pattern for producing plant hormones in <i>E. coli</i>, we introduced an incoherent feed forward loop (Mangan | + | In order to achieve the natural plants’ temporal pattern for producing plant hormones in <i>E. coli</i>, we introduced an incoherent feed forward loop (Mangan et al., 2006) to our circuit, including a new hybrid promoter part ([http://parts.igem.org/Part:BBa_K1139150 BBa_K1139150]). Plants produce their hormones transiently rather than steadily (Takei et al., 2001). Moreover, continuous overexpression of hormones is harmful to plants (K. Thiman, 1937). Thus, we thought that it should be important to achieve this transient temporal pattern for producing plant hormones in <i>E. coli</i>. Our designed system with an incoherent feed forward loop is shown in Fig. 2-3-16. We newly developed the <i>RM/lac</i> hybrid promoter, which is activated by CI and repressed by LacI (Fig. 2-3-17). We planned to ligate a hormone synthase part downstream of this hybrid promoter. Our mathematical model (Fig. 2-3-18) shows what the temporal pattern for plant hormone production should achieve. While the <i>rm/lac</i> hybrid promoter activation by CI is a single-step reaction, repression by LacI is a two-step reaction. Thus, the activation of <i>rm/lac</i> hybrid promoter is faster than its repression. This time lag between activation and repression is important for generating a temporal pattern of plant hormone production (details about our mathematical model can be found <font size ="5">[https://2013.igem.org/Team:Tokyo_Tech/Modeling/Incoherent_Feed_Forward_Loop#1._Introduction here]</font size>). |
</p> | </p> | ||
<gallery widths="350px" heights="200px" style="margin-left:auto; margin-right:auto;"> | <gallery widths="350px" heights="200px" style="margin-left:auto; margin-right:auto;"> | ||
Revision as of 09:03, 27 October 2013
Farming
Contents |
1. Introduction
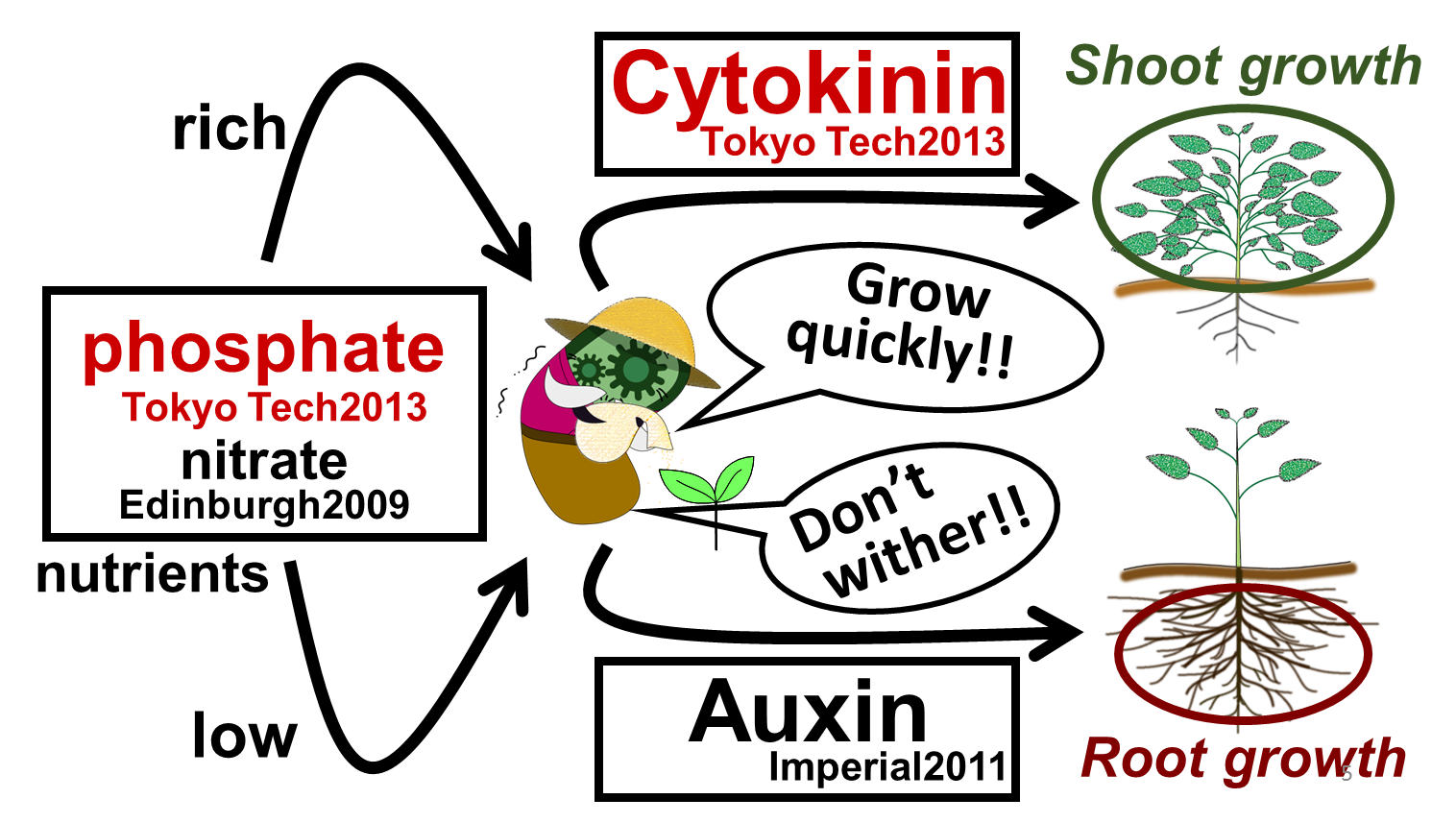
In our story, E. ninja retires from being an undercover warrior, and turns to farming (Fig. 2-3-1). Our improved part for phosphate sensor and a new part for cytokinin synthesis will lead us to our goal, which is to create “Farmer E. coli” that could increase plant growth by synthesizing several plant hormones depending on the soil environment. As for sensing soil nutrients, we focused on phosphate and nitrate because these are known to be especially important for plants. When nutrient levels are low, plants are in danger of withering. Therefore, we are proposing E. coli to produce the plant hormone “auxin,” which promotes the growth of plants’ roots (Fig. 2-3-2). Also, when the soil is rich in nutrients, plants can grow quickly. Therefore, we want to make E. coli produce the plant hormone "cytokinin," which promotes the growth of plants’ shoots.
Our "Farmer E. coli" can be implemented by combining our improved phosphate sensor part ([http://parts.igem.org/Part:BBa_K1139201 BBa_ K1139201]), our newly constructed cytokinin production part and two pre-existing parts which are the nitrate sensor part (Edinburgh 2009, [http://parts.igem.org/Part:BBa_K216005 BBa_K216005]) and the auxin production part (Imperial College 2011, [http://parts.igem.org/Part:BBa_K515100 BBa_K515100]) (Fig. 2-3-2). Since the existing phosphate sensor part (OUC-China 2012, [http://parts.igem.org/Part:BBa_K116401 BBa_K116401]) does not have sufficient data, we improved the part by using a different promoter. We also learned methods for quantitative analysis for cytokinin through bioassay of cucumber seed sprouts (Fig. 2-3-3) and through using ultra-performance liquid chromatography (UPLC).
In addition, to achieve the natural plants’ temporal pattern for producing plant hormones in E. coli, we introduced an incoherent feed forward loop to our circuit, including a new hybrid promoter part ([http://parts.igem.org/Part:BBa_K1139150 BBa_K1139150]). Our mathematical model for this system predicted that we can create temporal pattern for plant hormone production.
2. Phosphate dependent expression regulation
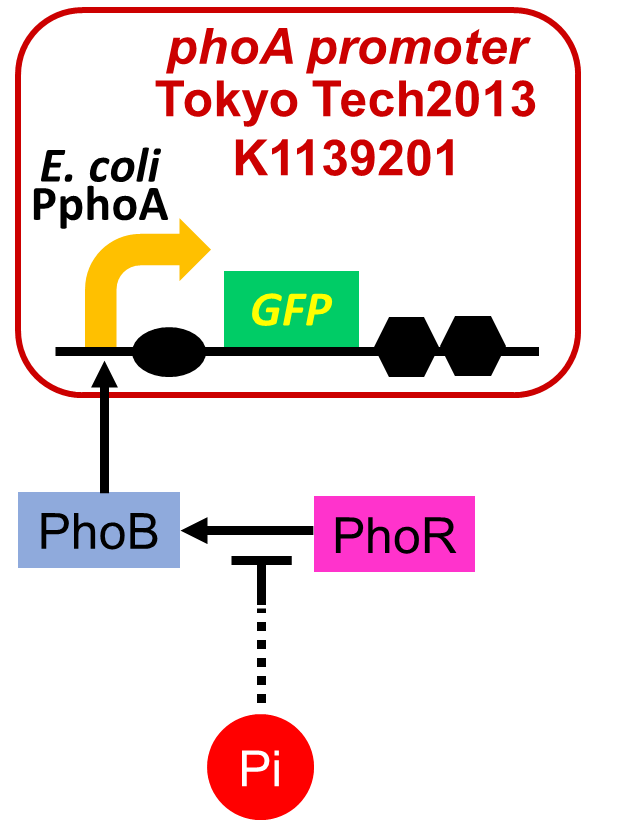
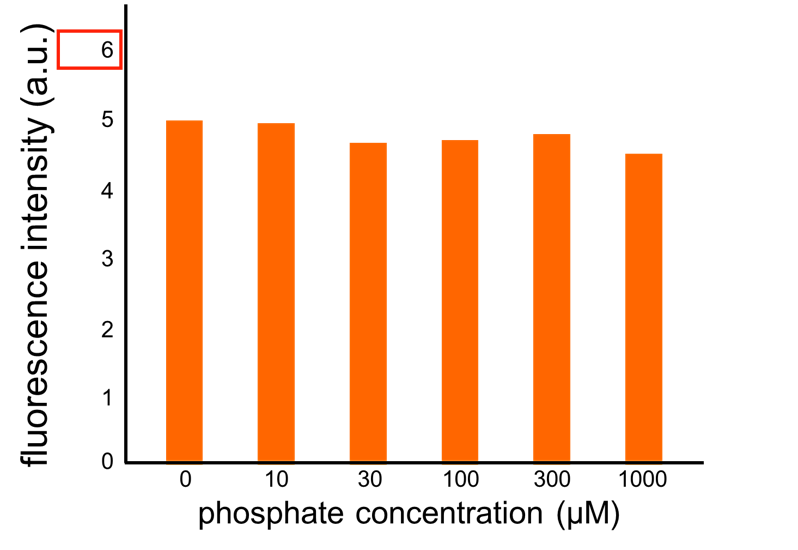
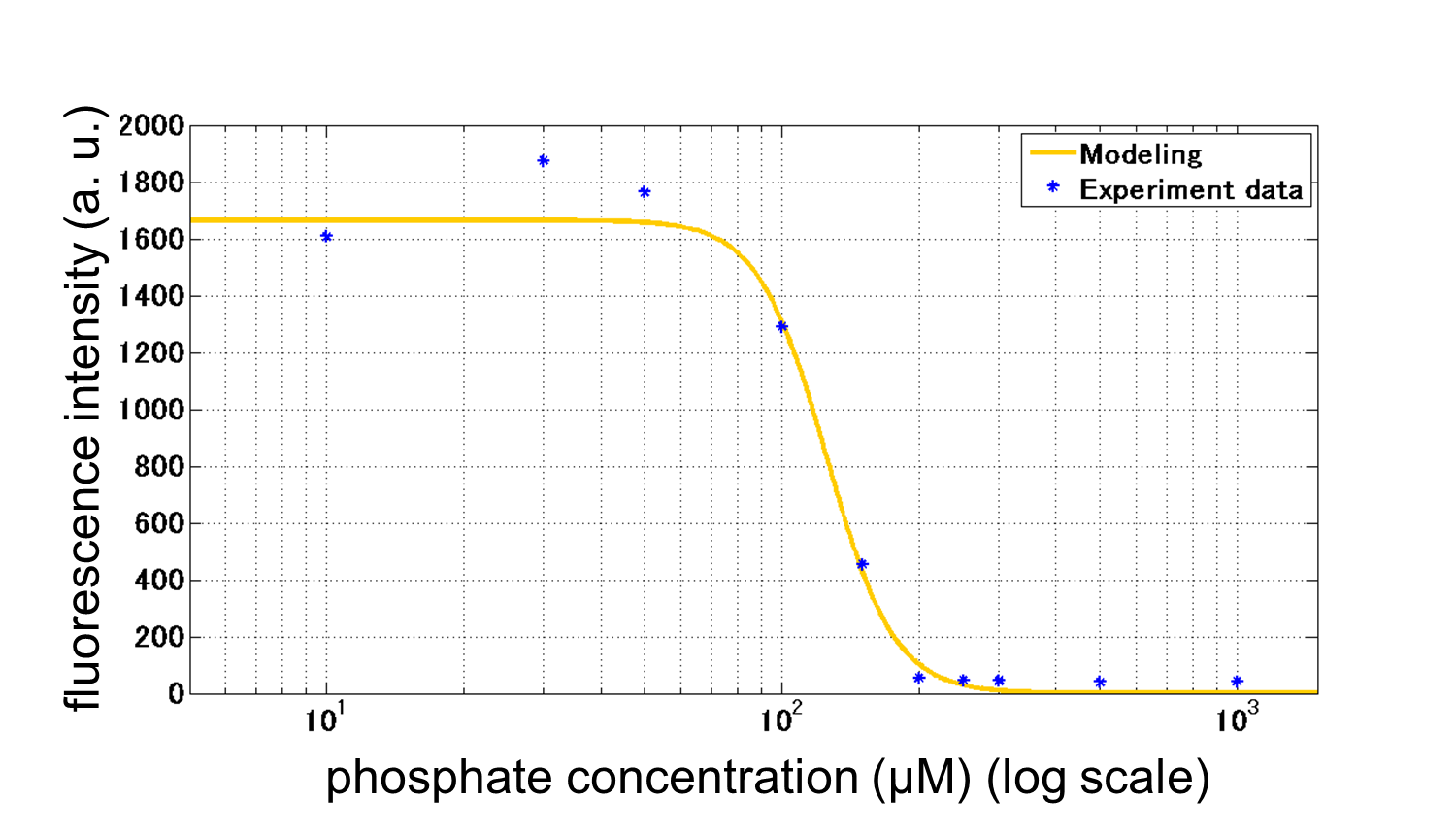
We improved a phosphate sensor part by using the inducible promoter of the alkaline phosphatase gene (phoA) from E. coli (M. Dollard et al., 2003). This promoter is repressed by high phosphate concentrations (Fig. 2-3-4). To know more about the mechanism of this promoter, please click here. We amplified the phoA promoter from E. coli (MG1655) and ligated this promoter into GFP part to construct the new part ([http://parts.igem.org/Part:BBa_K1139201 BBa_K1139201]). We then introduced this new part into E. coli (MG1655). Fig. 2-3-5 shows the result of our induction assay. It shows that the increase in phosphate concentration repressed the phoA promoter. Especially, we see that the phoA promoter is drastically repressed at phosphate concentrations of 100 to 200 microM. Though we also assayed OUC-China’s phosphate sensor part including phoB promoter ([http://parts.igem.org/Part:BBa_K116401 BBa_K116401]) by the same method as that for our phoA promoter assay, their part did not respond to the increase in phosphate concentration (Fig. 2-3-6). Thus, we concluded that we improved the phosphate sensor part (Note that the scales of the vertical axis are different between the two results). To know more about this assay, please see here.
From our results explained above, we determined parameters for the induction mechanism. By fitting the results to the following Hill equation (Fig. 2-3-7), we identified the parameters for the induction mechanism. α denotes the maximum GFP expression rate in this construct. m denotes the phosphate concentration at which the GFP expression rate is half of α. β denotes the hill coefficient. Those parameters (Fig. 2-3-8) can be used in our future modeling. Plants are reported to be in phosphate starvation when its concentration is below 1 mM (D. Hoagland et al., 1950). Our part can sense also the concentration below 1 mM (Fig. 2-3-9). Therefore, our improved part is useful for our farming circuit. Moreover, a sensor for phosphate concentration is valuable for various studies in synthetic biology.
3. Cytokinin synthesis
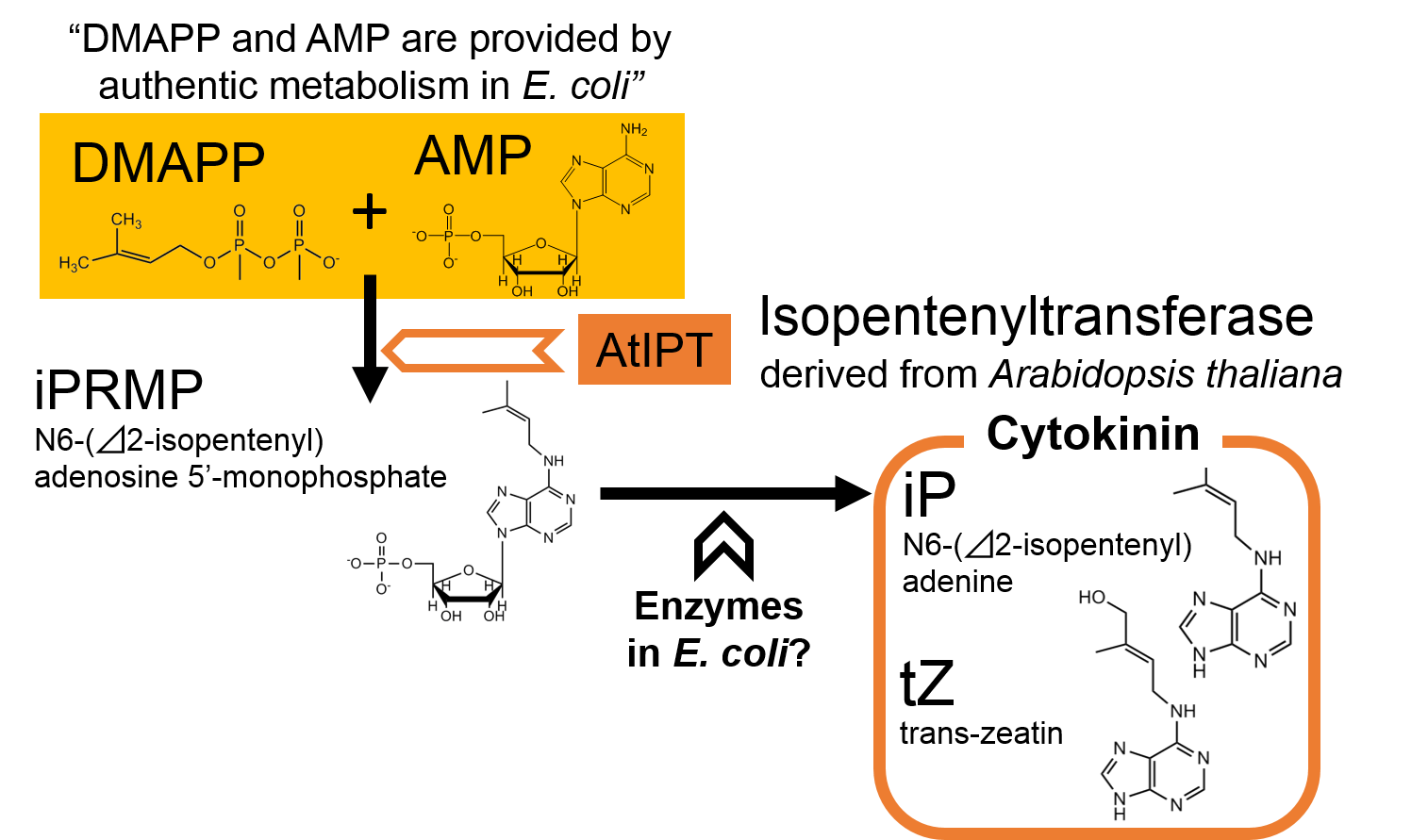
In order to construct cytokinin production part, we focused on AtIPT, a plant enzyme which catalyzes the synthesis of cytokinin. The mechanisms of cytokinin synthesis are shown in Fig. 2-3-10 (Takei et al., 2001). We ordered two DNA sequences of AtIPT (AtIPT4 and AtIPT7) derived from A. thaliana and constructed the part including one of them.
AtIPT, a plant enzyme, catalyzes the synthesis of iPRMP from DMAPP and AMP. According to the previous research, DMAPP, and AMP are provided by authentic metabolism in E. coli . It is also expected in the report that E. coli also has the enzymes which catalyze the synthesis of iP or tZ (both sorts of cytokinin) from iPRMP.
4. Quantitative analysis for cytokinin by bioassay
Before constructing genetic parts for cytokinin synthesis, we learned methods for quantitative analysis for cytokinin through a bioassay of cucumber seed sprouts (Fletcher et al., 1971; Porra et al., 1989) and through using ultra-performance liquid chromatography (UPLC).
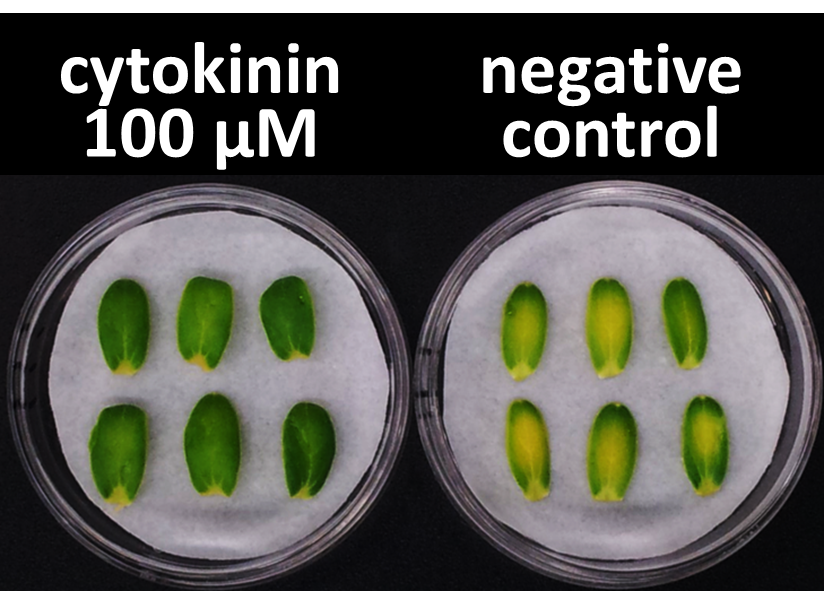
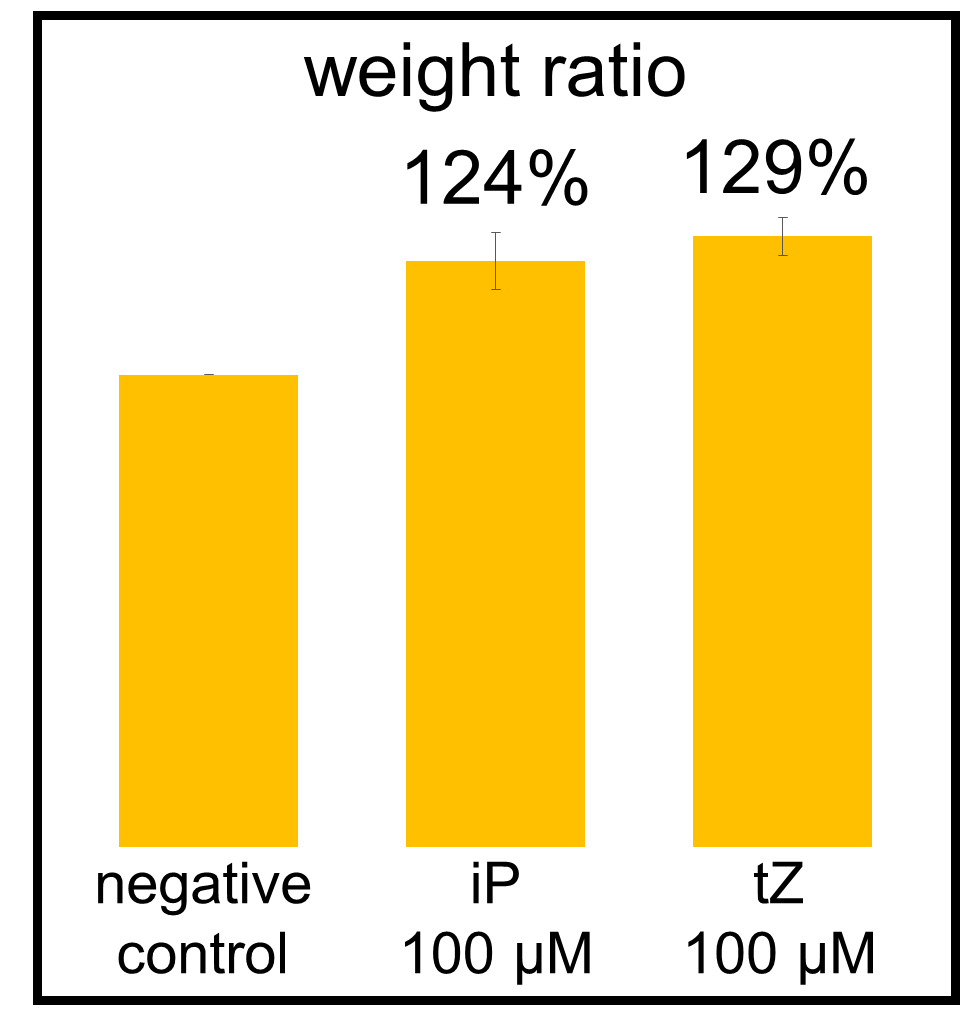
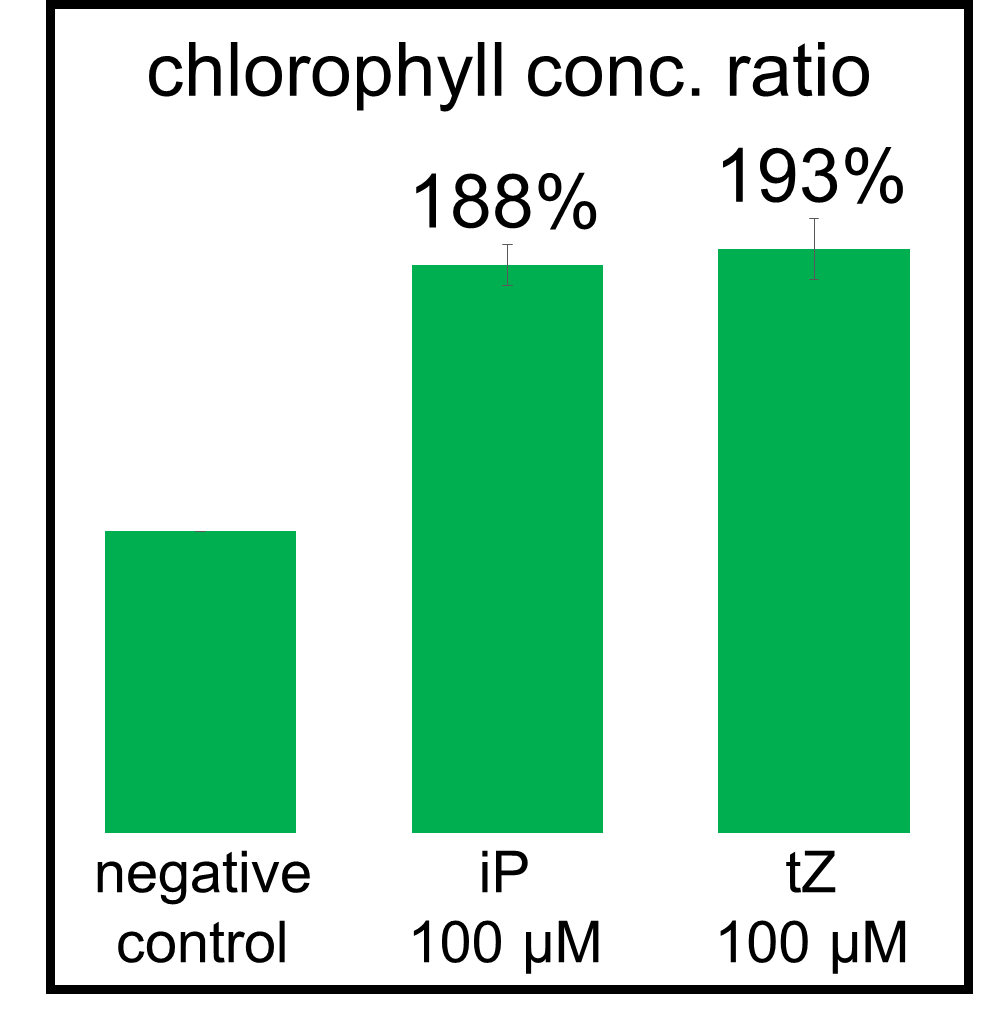
In our bioassay of cucumber seed sprouts, we learned how to detect cytokinin and found which concentration of cytokinin can act on plants. We planted the seeds and germinated for 5 days. Then, we cultivated the sprouts in standard cytokinin sample solutions. After 24 hours in the dark and 24 hours in the light, we measured the weight of the sprouts and also homogenized them to measure the concentration of chlorophyll (Fig. 2-3-12). Fig. 2-3-11 shows the result that the sprouts in cytokinin solution are larger and greener than the negative controls, which are the sprouts in solution without cytokinin. The quantitative results (Fig. 2-3-13 and Fig. 2-3-14) also show that cytokinin increases the weight of the sprouts and the concentration of chlorophyll. To know more about this assay, please see here.
iP : 6-(γ, γ-Dimethylallylamino) purine, tZ : trans-zeatin
The absorbances of the supernatant were read at 663.6 and 646.6 nm. Calculation of concentrations of the chlorophyll were carried out as described by Porra et al.,1989.
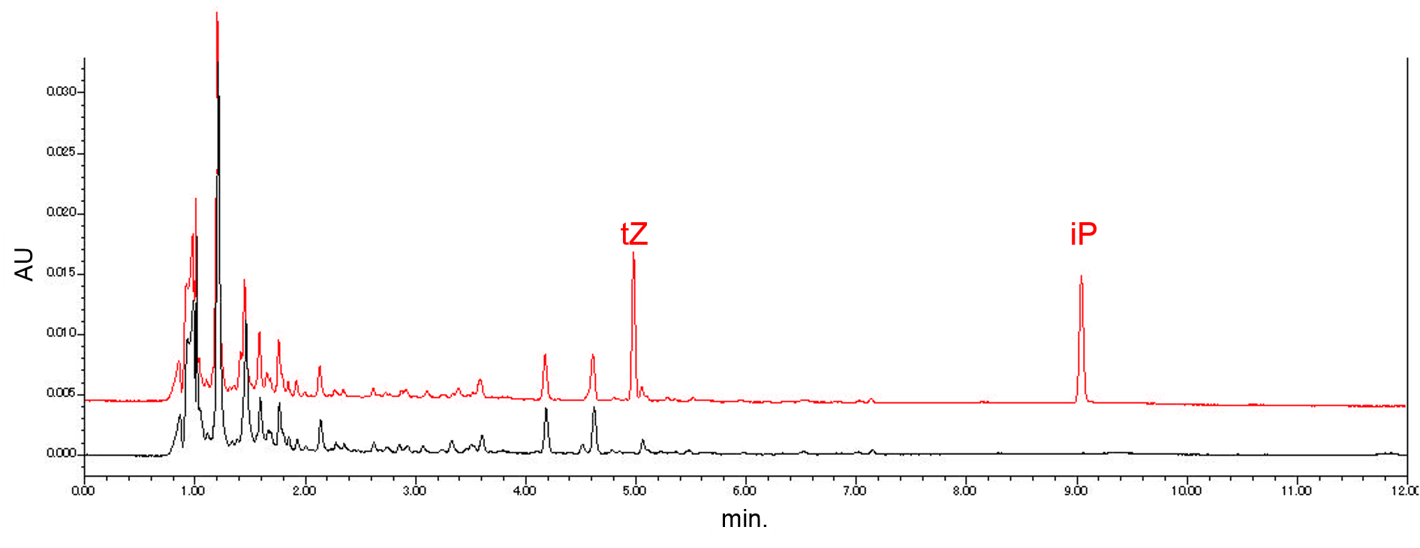
Through ultra-performance liquid chromatography (UPLC), we planned to confirm that our "Farmer E. coli" actually produce cytokinin. Before completing the system to produce cytokinin in E. coli, we determined the retention times of authentic samples of iP and tZ by using UPLC. Fig. 2-3-15 shows the result. In addition, we confirmed that iP and tZ were able to be detected from the mixture of the E. coli culture medium and the cytokinin solution. To see the detail method of this experiment, please click here.
5. Hybrid promoter for temporal pattern generation
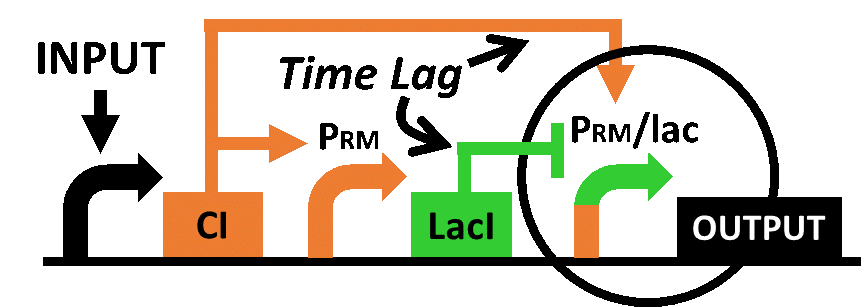
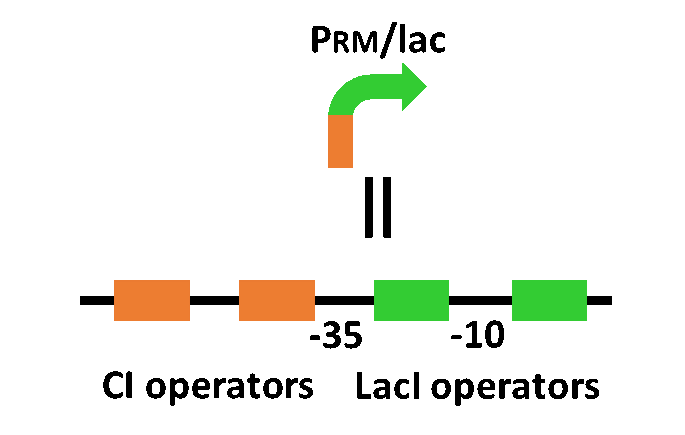
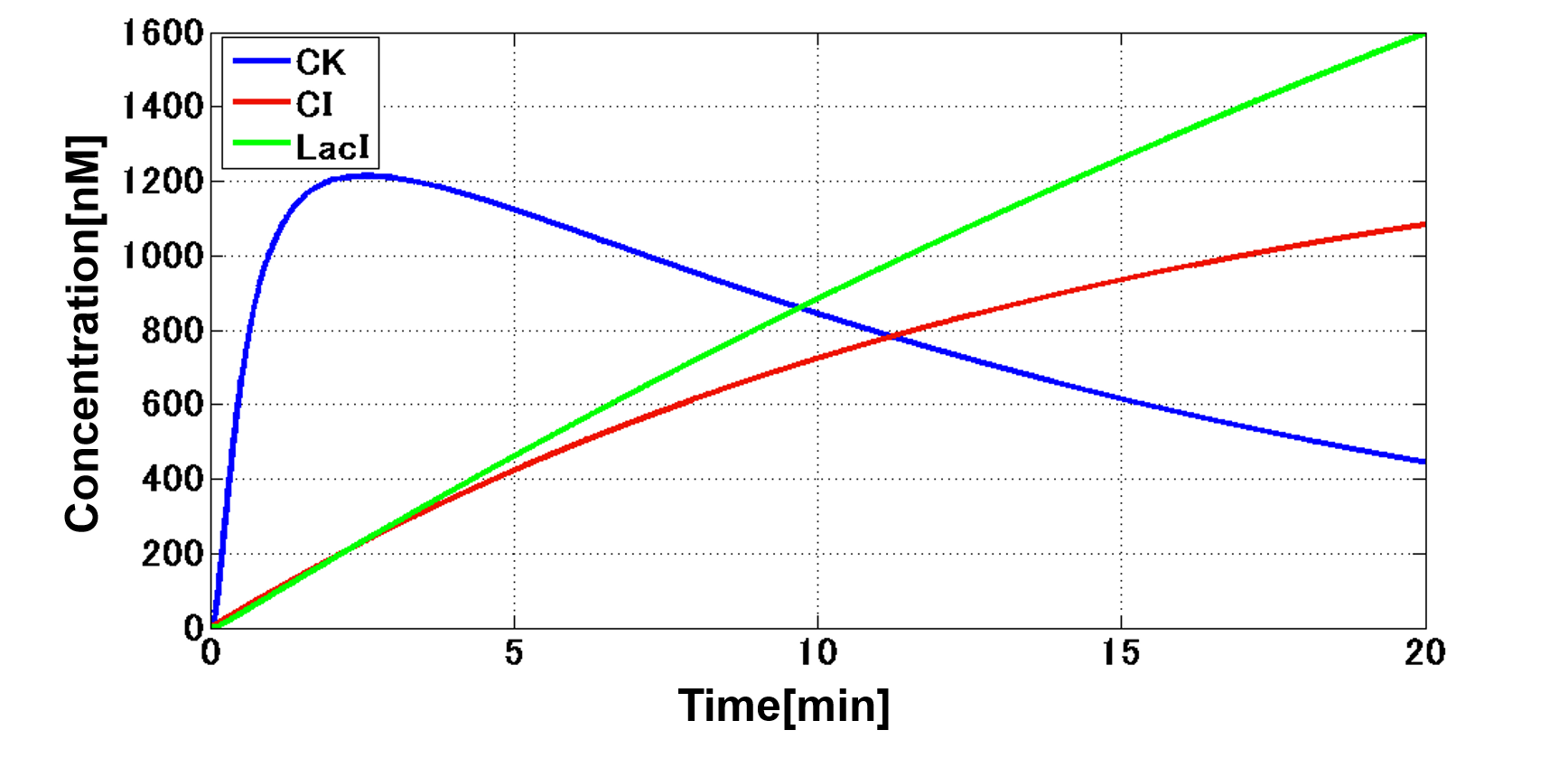
In order to achieve the natural plants’ temporal pattern for producing plant hormones in E. coli, we introduced an incoherent feed forward loop (Mangan et al., 2006) to our circuit, including a new hybrid promoter part ([http://parts.igem.org/Part:BBa_K1139150 BBa_K1139150]). Plants produce their hormones transiently rather than steadily (Takei et al., 2001). Moreover, continuous overexpression of hormones is harmful to plants (K. Thiman, 1937). Thus, we thought that it should be important to achieve this transient temporal pattern for producing plant hormones in E. coli. Our designed system with an incoherent feed forward loop is shown in Fig. 2-3-16. We newly developed the RM/lac hybrid promoter, which is activated by CI and repressed by LacI (Fig. 2-3-17). We planned to ligate a hormone synthase part downstream of this hybrid promoter. Our mathematical model (Fig. 2-3-18) shows what the temporal pattern for plant hormone production should achieve. While the rm/lac hybrid promoter activation by CI is a single-step reaction, repression by LacI is a two-step reaction. Thus, the activation of rm/lac hybrid promoter is faster than its repression. This time lag between activation and repression is important for generating a temporal pattern of plant hormone production (details about our mathematical model can be found here).
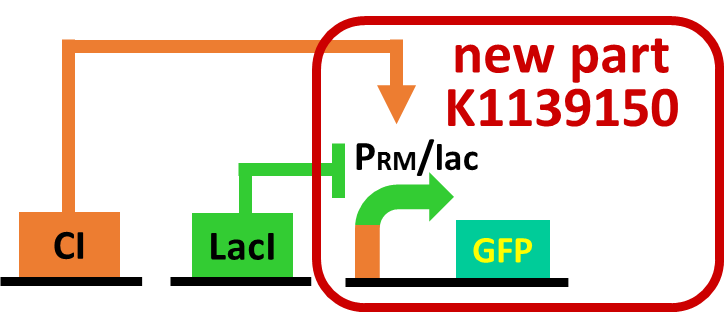
As a first step to achieve this incoherent feed forward loop, we constructed the circuit shown in Fig. 2-3-19 to confirm that our new rm/lac hybrid promoter actually works. We set GFP as the output of rm/lac hybrid promoter and introduced the circuit into E. coli . We have already confirmed that our new rm/lac hybrid promoter is actually activated by CI through an induction assay (Fig. 2-3-20). This part has been submitted ([http://parts.igem.org/Part:BBa_K1139150 BBa_K1139150 new part]). Details about this assay can be found here. We are now assaying induction in response to combinations of CI and LacI.
6. Applications
Our project will be applied to studying the plants’ response to external plant hormones. We programmed an incoherent feed forward loop to our new device of plant hormone production. By applying the incoherent feed forward loop, we can achieve pulse-generation of the output (Basu. S et al., 2003). Moreover, the pulse wave can be customized with changing the parameters, for example, intensity of promoters and various interactions. Utilizing the incoherent feed forward loop, we aim to construct artificial pulse-generator circuits which can output plant hormones in various pulse waves. Various artificial genetic circuits which can change the output chronologically, including incoherent feed forward loop, have been reported (Elowitz et al., 2000; Eileen et al., 2005). By combining plant hormone synthesis genes to these artificial genetic circuits, we will be able to make E. coli produce plant hormones in chronological patterns. Analyzing the response of plants to plant hormones given in temporal change leads to elucidate not only the sensitivity of plants’ response to plant hormones but also the abilities of plants in signal transduction. Therefore, our project contributes to constructing the pulse-generator for plant hormones using E. coli. Findings in plant science using this strategy must be important for contributing to agriculture and furthermore solving worldwide food shortages. We hope to meet public expectations by realizing agriculture aid by bacteria, though there may be some difficulties.
 "
"