Team:Newcastle/Modelling/L-form Switch
From 2013.igem.org
| Line 2: | Line 2: | ||
==L-form switch== | ==L-form switch== | ||
| - | Although it has been documented that L-forms can be produced by a specific mutation in the ''murE'' gene, we strongly suspected that preventing the expression of the entire ''murE'' operon would have the same effect. However we were not certain, and therefore modelled the biosynthesis of peptidoglycan, the primary component of the cell wall, using BioNetGen. Modelling was based | + | Although it has been documented that L-forms can be produced by a specific mutation in the ''murE'' gene, we strongly suspected that preventing the expression of the entire ''murE'' operon would have the same effect. However we were not certain, and therefore modelled the biosynthesis of peptidoglycan, the primary component of the cell wall, using BioNetGen. Modelling was based on the [http://www.genome.jp/kegg-bin/show_pathway?map00550 KEGG pathway] for peptidoglycan synthesis in ''B. subtilis''. The model put the operon under the control of a xylose inducible promoter, and demonstrated that at low xylose concentrations (and thus no expression of the ''murE operon'') no peptidoglycan was produced. In the presence of xylose peptidoglycan synthesis did occur. As we were not worried about the quantity of peptidoglycan that was produced, just whether it was, arbitrary unit were used for peptidoglycan production. This was also because quantifying peptidoglycan is non-trivial. |
These results gave the team confidence that the switch BioBrick we designed would work as desired. Experimental results later validated this. | These results gave the team confidence that the switch BioBrick we designed would work as desired. Experimental results later validated this. | ||
Revision as of 18:52, 28 October 2013

L-form switch
Although it has been documented that L-forms can be produced by a specific mutation in the murE gene, we strongly suspected that preventing the expression of the entire murE operon would have the same effect. However we were not certain, and therefore modelled the biosynthesis of peptidoglycan, the primary component of the cell wall, using BioNetGen. Modelling was based on the [http://www.genome.jp/kegg-bin/show_pathway?map00550 KEGG pathway] for peptidoglycan synthesis in B. subtilis. The model put the operon under the control of a xylose inducible promoter, and demonstrated that at low xylose concentrations (and thus no expression of the murE operon) no peptidoglycan was produced. In the presence of xylose peptidoglycan synthesis did occur. As we were not worried about the quantity of peptidoglycan that was produced, just whether it was, arbitrary unit were used for peptidoglycan production. This was also because quantifying peptidoglycan is non-trivial. These results gave the team confidence that the switch BioBrick we designed would work as desired. Experimental results later validated this.
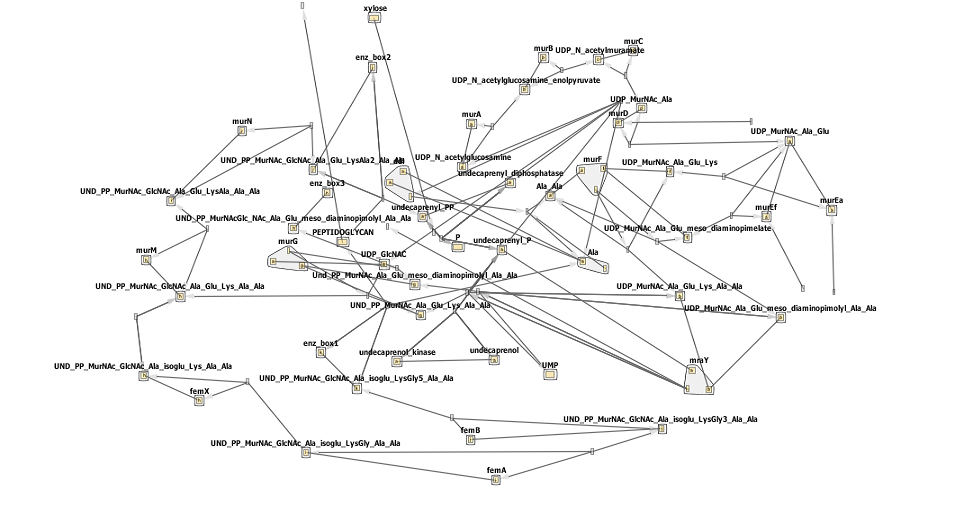
The biosynthesis of peptidoglycan is quite complicated, with one initial pathway splitting into 3, each fork resulting in peotidoglycan being formed. This can be seen by the network below- don't worry if it looks confusing! Synthesis can be broadly defined in a few steps steps.
- In the first step fructose 6-phosphate is converted to glucosamine-6-phosphate.
- Then an acetyl group is donated, creating N-acetyl-glucosamine-6-phosphate.
- This is subsequently isomerized to N-acetyl-glucosamine-1-phosphate.
- UTP reacts with this N-acetyl-glucosamine-1-phosphate forming UDP-N-acetylglucosamine. This is where the KEGG pathway, and our model, starts.
- UDP-N-acetylglucosamine is converted to UDP-N-acetylmuramic acid, and this undergoes multiple rounds of amino acid addition. It is these steps, indicated on the pathway below, that are governed by a few of the murE operon genes- murD, murE, murF,murG and mraY. The switching off of these genes prevents the addition to UDP-MurNAc-L-Ala of more amino-acids and thus prevent peptidoglycan biosynthesis.
- With a functional murE UDP-MurNAc pentapeptide is formed.
- This precursor then passes from the cytosol to the cytoplasmic membrane, and is converted to the lipid PP-MurNac penta.
- After a few more reactions a disaccharide is formed and added to a long glycan chain. One transglycoslyation later and peptidoglycan has been increased.
Results
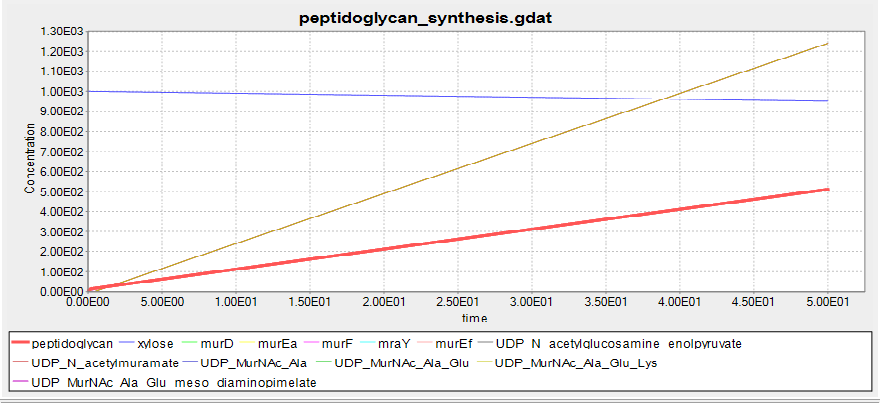
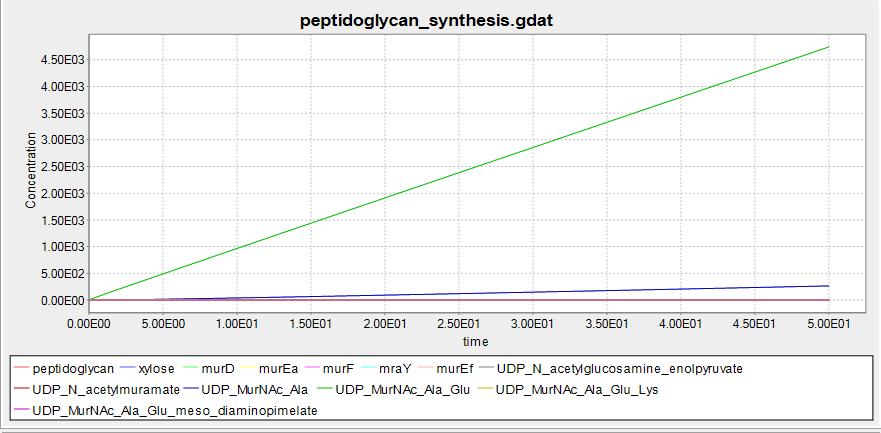
These are the results of simulating the model. The units of concentration on the y-axis are arbitrary, as are the units of time on the x-axis. These results suggest that B. subtilis cells that have been transformed with our BioBrick will be able to grow a cell wall if there is xylose present, but not without.
The model below represents peptidoglycan biosynthesis with xylose present:
The graph is below is from the simulation at 0mM xylose. No peptidoglycan is synthesised, suggesting that the BioBrick we have designed will function as desired. Note that, unlike the graph above, no UDP_murNAc_Ala_Glu_Lys is being synthesised. This is the product of the reaction murE catalyses- this suggests that it is the switching off of the murE operon genes causing the lack of peptidoglycan synthesis.
The circled enzymes are coded by genes found in the murE operon. As can be seen from the [http://www.genome.jp/kegg/pathway/map/map00550.png KEGG pathway] above these enzymes are at a branching point, with both subsequent sub-pathways synthesising peptidoglycan. So the switching off of a specific enzyme further along the pathway wouldn't prevent synthesis. Many of the enzymes upstream of murE are involved in other pathways, so preventing the synthesis of these enzymes would have other, potentially harmful side-effects. Therefore the murE operon is the perfect area to target for controlling cell wall synthesis.
This model is comprised of a vast number of reactions and substrates/enzymes, many of which have not been characterized. We were able to find parameters for the catalytic rates of murD, murE and murF, although not for B. subtilis. As these rates are similar, we extended these values to other enzymes in the pathway. Whilst this may not be fully accurate we aren't attempting to intricately quantify the amount of peptidoglycan, and so should not be a problem. Substrates involved in but not formed by the peptidoglycan biosynthesis pathway were kept high so as not to be rate limiting, and were chosen as the concentrations at which the enzymes best functioned in vivo as described by [http://link.springer.com/article/10.1007/s13238-010-0132-9?no-access=true Bhakta et. al].
Enzyme concentrations were set to a few millimolar and the simulation was done as an differential equations, as we were mostly interested in the model's qualitative behaviour. The The BioNetGen file (in .txt format, if you'd like to try it out just change the extension to .bngl)
Below is the network diagram for this model. It is very complicated so don't worry if you don't understand all of it!
References
[http://link.springer.com/article/10.1007/s13238-010-0132-9?no-access=true Basavannacharya C., Moody P., Munshi T., Cronin N., Keep N., Bhakta S. (2010) Essential residues for the enzyme activity of ATP-dependent MurE ligase from Mycobacterium tuberculosis. Protein & Cell 11,1011-1022]
[http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0060143 Munshi T, Gupta A, Evangelopoulos D, Guzman JD, Gibbons S, et al. (2013) Characterisation of ATP-Dependent Mur Ligases Involved in the Biogenesis of Cell Wall Peptidoglycan in Mycobacterium tuberculosis. PLoS ONE 8(3): e60143. doi:10.1371/journal.pone.0060143]
[http://www.ncbi.nlm.nih.gov/pubmed/19212404 Leaver M., Dominguez-CuevasP., Coxhead J.M., Daniel R.A. and Errington J. (2009) Life without a wall or division machine in Bacillus subtilis. Nature, 457, 849-853.]
 "
"